
Abstract
Aims: Multiple surgical revisions are often necessary in individuals with congenital heart defects affecting the RVOT or pulmonary valve. There are no multicentre data on the feasibility and safety of percutaneous pulmonary valve implantation (PPVI) using the SAPIEN 3 (S3) transcatheter heart valve. The aim of this study was to explore the short-term safety, feasibility, and haemodynamic outcomes of PPVI using the S3 transcatheter heart valve.
Methods and results: Pulmonic S3 is an observational registry of patients undergoing PPVI with the S3 valve across centres in Europe and Canada. Data for 82 patients (mean age 27.3 years) were obtained. The most common underlying diagnosis was tetralogy of Fallot (ToF) (58.5%), with 16.0% of patients having native RVOT anatomy; 90.2% received pre-stenting. Prosthesis dislodgement occurred in one patient and conduit perforation in another. Both were successfully resolved without the need for open surgery. Peak systolic gradient over the RVOT fell from 46.3 mmHg to 17.2 mmHg, moderate/severe pulmonary regurgitation from 86.3% to 0.0%, and NYHA ≥II from 86.0% to 15.2%. During follow-up, valve thrombosis was observed in two patients which resolved with adequate anticoagulation. No other procedural complications, endocarditis, stent fracture or death were reported within two years.
Conclusions: PPVI with the S3 valve appears feasible and safe in a wide range of patients with congenital heart defects, with good short-term haemodynamic and functional outcomes. ClinicalTrials.gov Identifier: NCT02777892
Abbreviations
AT: anaerobic threshold
PA: pulmonary atresia
PPVI: percutaneous pulmonary valve implantation
PV: pulmonary valve
RVOT: right ventricular outflow tract
RVSP: right ventricular systolic pressure
TA: truncus arteriosus
ToF: Tetralogy of Fallot
VO2: peak oxygen consumption
Introduction
Many congenital heart defects, such as tetralogy of Fallot (ToF), pulmonary atresia (PA), and truncus arteriosus (TA), are characterised by pulmonary valve (PV) and/or right ventricular outflow tract (RVOT) defects1. The consequences of such dysfunctional anatomy include PV regurgitation, insufficient blood flow to the lungs, and poorly oxygenated blood in the systemic circulation. As such, surgical reconstruction of the RVOT using patches, conduits and biological valves (homografts, bioprostheses, or xenografts) is necessary2. Unfortunately, such structures tend to undergo a functional decline during the patient’s lifetime, owing to physical degeneration, calcification, and/or a lack of adaptation to growing native anatomy2,3. A patient is therefore likely to require multiple repeat operations, each resulting in greater scarring of cardiac and vascular tissue and carrying an ever higher surgical risk4. Furthermore, open heart surgery is associated with considerable psychological strain, patient morbidity and lengthy recovery times4-6.
Percutaneous pulmonary valve implantation (PPVI) is an emerging, minimally invasive procedure that aims to extend the lifespan of an existing RVOT revision, in order to delay the need for repeat surgical intervention7. In select patients, it may also be an appropriate first-line approach to PV replacement8. In addition to a relatively low incidence of major complications and satisfactory survival rates9, studies have shown PPVI to hold the potential for significant cost savings, shorter hospital stays and a lesser loss of wages10. However, most of the current evidence for PPVI has been gathered using the earlier-generation Melody™ (Medtronic, Minneapolis, MN, USA) or Edwards SAPIEN (Edwards Lifesciences, Irvine, CA, USA) valves9,11. With the availability of the SAPIEN 3 (Edwards Lifesciences), paravalvular leak is minimised through inclusion of an outer polyethylene terephthalate skirt12,13. While use of this valve and its deflectable Commander delivery system has been shown to reduce the rate of several pertinent complications in transcatheter aortic valve implantation (TAVI)12, there are currently few formal data on S3 implantation in the pulmonic position.
The objective of the present registry (Pulmonic S3) analysis was to explore the short-term safety, feasibility, and haemodynamic outcomes of PPVI using the S3 transcatheter heart valve.
Methods
The Pulmonic S3 registry was initiated by investigators in March 2016. It is an ongoing, multicentre, observational study on patients undergoing PPVI with the S3 technology. Patients who had already received an S3 valve in the pulmonic position were retrospectively enrolled and prospectively followed from the time of inclusion onwards. The study was approved by the relevant ethics committee at each centre prior to patient documentation and performed in accordance with the Declaration of Helsinki and its amendments. All patients provided data release forms and their written informed consent to participate.
CENTRES
Based on information provided by Edwards Lifesciences and the core group of participating sites, we estimated that, at the time of project initiation, approximately 11 centres globally (excluding the USA) had performed at least one PPVI using S3 technology. Each was invited to participate in the study. No minimum requirements pertaining to the number of implants or operator experience were specified.
PATIENTS
Inclusion criteria were a clinical indication for PPVI and prior implantation of the S3 transcatheter heart valve in the pulmonic position, with the decision to perform the procedure taken independently and prior to registry inclusion. In order to represent the broadest possible spectrum of patients undergoing PPVI, no further inclusion/exclusion criteria were stipulated.
DATA COLLECTION
Given the wide range of patients represented in the study, the Pulmonic S3 electronic case report form was designed to accommodate the broadest possible spectrum of clinical settings in which PPVI may be indicated. As such, the data collected reflect the site’s standard procedures. Data were subjected to regular automatic and manual checks for plausibility and completeness. No core lab was established to confirm the clinical variables collected (such as echo) at the centres.
OUTCOMES AND DEFINITIONS
Feasibility was assessed according to procedural success (defined as successful implantation of a single valve at the intended location and retrieval of the delivery catheter). Safety outcomes included the rates of periprocedural technical complications (device malfunction [defined as failure to meet performance specifications or otherwise perform as expected], valve dislocation requiring surgical intervention, and stent dislocation), and periprocedural adverse events (significant bleeding; arrhythmia requiring pacing, drugs or cardioversion; conduit/RVOT rupture requiring emergency stenting or surgery; significant neurological impairment based on clinical assessment; coronary compression; myocardial infarction; pulmonary embolism; periprocedural death).
STATISTICAL ANALYSIS
Data are presented as descriptive data summaries. Categorical variables are expressed as absolute numbers and frequencies, while continuous variables are expressed as means with standard deviations, median with interquartile range and minimum–maximum data ranges. All analyses were performed using SPSS Statistics, Version 24.0 (IBM Corp., Armonk, NY, USA).
Results
PATIENT CHARACTERISTICS
In total, 82 patients underwent PPVI using S3 technology at eight sites (range one to 28 patients). The majority of patients were male (59.8%), with a mean age of 27.3±14.7 years (range: 5–61 years) (Table 1). The most common underlying diagnosis was ToF (58.5%), followed by PA without ventricular septal defect (12.2%). Very few patients had a native RVOT anatomy prior to PPVI (16.0%); transannular patch was the most common residual from prior RVOT revision (25.9%), followed by a stentless xenograft and other stented bioprosthesis (both 21.0%), and homograft (13.6%). Most patients were in NYHA Class II (38.6%) or III (47.4%) at baseline.
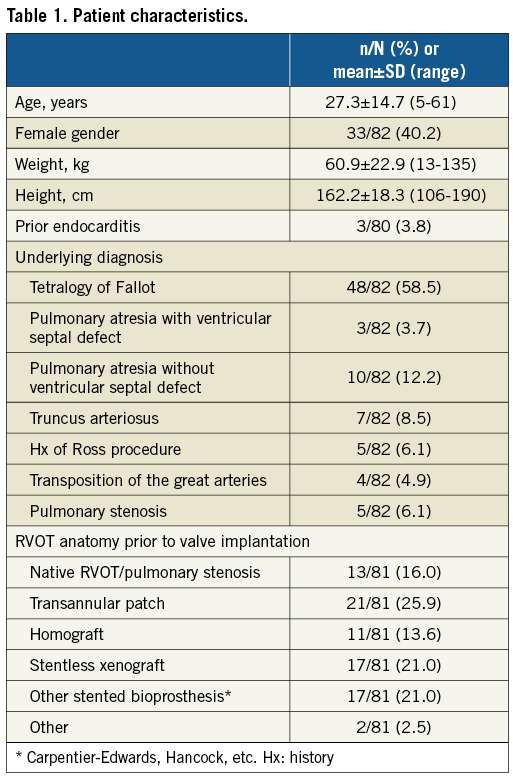
PROCEDURAL CHARACTERISTICS
The majority of PPVI procedures were carried out via the transfemoral access route (93.9%), with general anaesthesia employed in 63.0% (Table 2). RVOT pre-stenting was performed in 90.2%, either at the time of the procedure or prior to the day of the procedure (both 50.0%), with the majority receiving one stent only (70.4% overall). Eighty point two percent (80.2%) of valves underwent balloon predilation prior to prosthesis landing, with the mean minimal diameters before and after predilation being 18.3±5.1 and 24.1±3.0 mm, respectively. No 20 mm S3 valves were implanted, while 23 mm, 26 mm and 29 mm valve implantation was fairly evenly distributed across patients (39.0%, 29.3% and 31.7%, respectively). The mean procedural time was 99.9 minutes, with a mean fluoroscopy time of 63.4 minutes and a mean contrast agent volume of 162.2 mL.
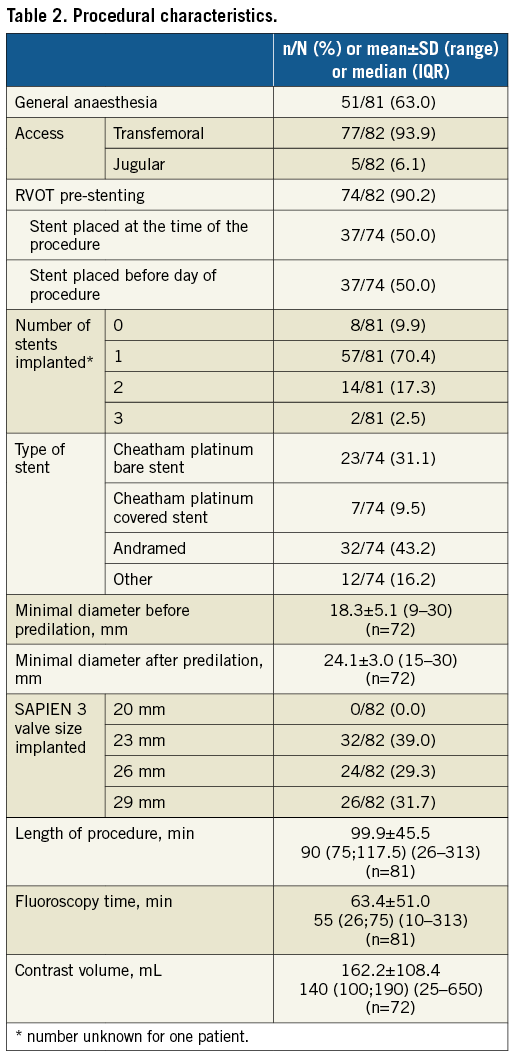
PERIPROCEDURAL OUTCOMES AND SAFETY
Procedural success was achieved in 81 out of 82 patients (Table 3). The one unsuccessful procedure was performed in a 22-year-old male patient with an underlying diagnosis of ToF (valve size implanted: 23 mm) where a technical complication during the first attempt at implantation via the transfemoral route was experienced (prosthesis dislodgement on the balloon with difficulty removing the undeployed valve from the superior vena cava); however, a repeat procedure via the jugular access route was successful. Another patient (male, aged 29 years with an underlying diagnosis of TA; valve size implanted: 26 mm; existing conduit type: homograft) experienced conduit perforation during covered stent placement with extravasation of contrast into the pericardium. This was resolved by placing a second covered stent with no contrast extravasation. The patient had an uneventful follow-up. No further procedural complications were reported.
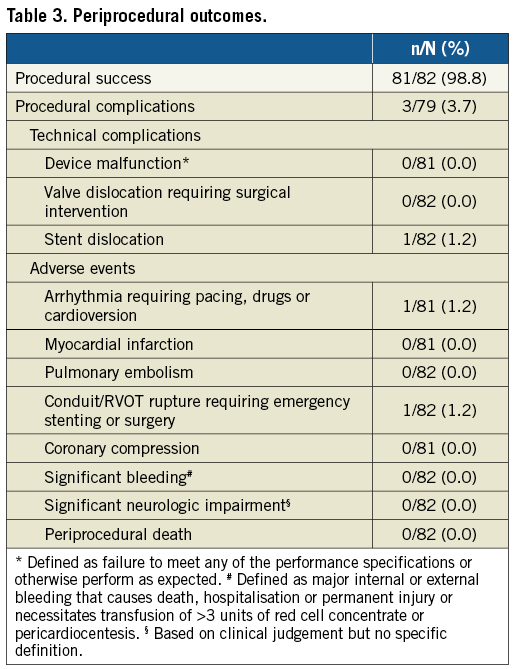
COMPLICATIONS DURING FOLLOW-UP
During follow-up (median 201 days; IQR 69 to 365 days), two patients with pulmonary valve thrombosis were reported. The first patient (female, aged 59, underlying diagnosis ToF, valve size implanted 29 mm) with suspected thrombus while not on acetylsalicylic acid (ASA) was diagnosed two months after the procedure using echocardiography and computed tomography (CT) scan (no clinical symptoms); this resolved during three months of coumadin therapy. The second patient (female, aged 18 years, underlying diagnosis ToF, valve size implanted 29 mm), on ASA, with leaflet thrombosis diagnosed six months after PPVI was diagnosed based on echocardiography (no clinical symptoms, but increasing gradients) and successfully treated with low-molecular heparin for two months, with complete resolution of thrombosis reported. No other events such as endocarditis, stent fracture or mortality due to any cause were reported throughout follow-up.
FUNCTIONAL OUTCOMES
By post-procedural day 30, the peak systolic gradient over the RVOT had fallen from its preprocedural mean of 46.3 mmHg to just 17.2 mmHg, slightly increasing at six and 12 months (Figure 1). While 86.3% of patients had pulmonary regurgitation that was moderate/severe at baseline, no patients displayed >mild pulmonary regurgitation after PPVI, with its being absent or trivial in the majority of patients thereafter (Figure 2A). A similar trend was observed for the tricuspid valve, with right ventricular systolic pressure falling from 47.8 mmHg at baseline to 29.3 mmHg and a slight increase thereafter, and the proportion of patients with moderate/severe tricuspid regurgitation falling from 29.6% to 8.8% (Figure 2B). Both VO2 and anaerobic threshold (AT) appeared to increase nominally after PPVI, though very few data were available for these variables (Table 4). The proportion of patients with an NYHA Class of ≥II fell from 86.0% at baseline to 15.2% at 30 days, with no patients being in NYHA Class ≥II at one year (Figure 3).
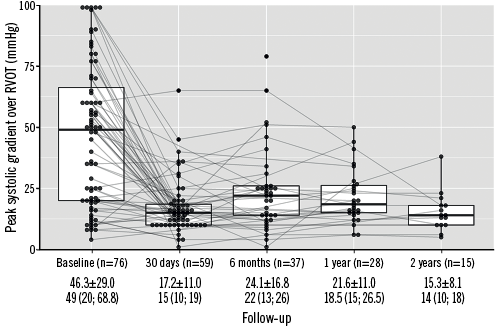
Figure 1. Peak gradient over RVOT (mmHg) over time. Values in means±SD and medians (interquartile range). RVOT: right ventricular outflow tract
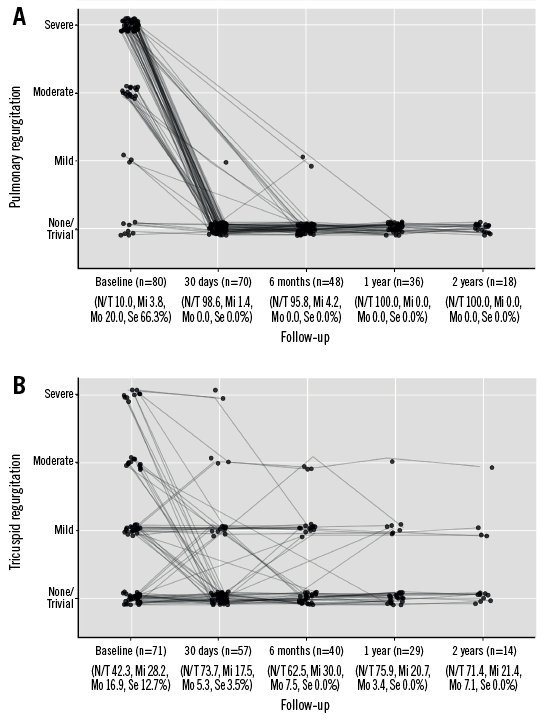
Figure 2. Pulmonary and tricuspid regurgitation over time. A) Pulmonary regurgitation. B) Tricuspid regurgitation.
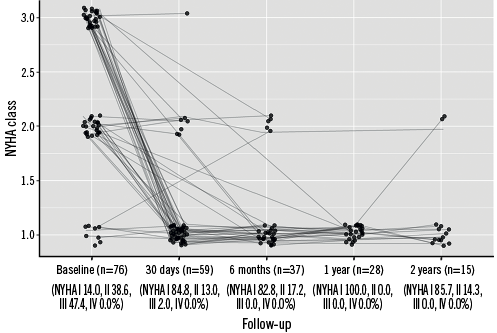
Figure 3. New York Heart Association classification over time. NYHA: New York Heart Association
Discussion
Pulmonic S3 is the first multicentre registry on patients undergoing PPVI with the S3 valve. The present analysis provides evidence for its feasibility and early safety across a wide range of patients with a variety of congenital heart defects, RVOT anatomies and ages. Complications were rare, with haemodynamic and functional improvements that appear to persist during the year after the procedure. These novel data pave the way for confident use of the new-generation S3 valve technology within the PPVI setting.
FEASIBILITY
The feasibility of PPVI with the S3 valve was first reported by Rockefeller et al in 2016 in a single subject with severe pulmonary regurgitation following ToF repair14. Since then, there has been a dearth of publications on the subject. We herein provide data on a relatively large number of patients in whom PPVI with the S3 was successfully performed, regardless of age, underlying congenital defect, and preprocedural RVOT anatomy. While current indications for PPVI typically include dysfunctional RVOT with surgically placed conduits and bioprosthetic valve failure8, there is movement towards its use in select patients with native RVOT morphology15. Indeed, in the present registry, 16.0% of those enrolled had such native anatomy, and all were successfully treated with S3 PPVI. Furthermore, a third of patients received a 29 mm prosthesis, which is only available for the newer-generation SAPIEN valves. In a similar registry on PPVI with the SAPIEN XT valve (Edwards Lifesciences), Pulmonic XT, only 17.4% of patients received a 29 mm prosthesis11. Besides patient heterogeneity, this may have been influenced by the relatively larger catheter size required for delivery of the 29 mm XT valve, thus limiting implantation in patients with smaller or less compatible access vessels. The S3 design permits smaller crimping, smaller delivery sheaths, and consequently delivery of the 29 mm valve in patients who may previously have been considered unsuitable13. As such, a greater variety of patients is able to benefit from PPVI with the S3 technology12.
HAEMODYNAMIC AND FUNCTIONAL IMPROVEMENTS
As reported for PPVI with earlier SAPIEN technologies11,16,17, use of the S3 resulted in substantial reductions in both pulmonary regurgitation and pressure gradients over the RVOT. However, while 5% of patients undergoing PPVI with the SAPIEN XT were reported still to have clinically relevant (moderate/severe) pulmonary regurgitation after the procedure11, this was not the case for any of the present S3 recipients, possibly partly attributable to the S3’s outer polyethylene terephthalate cuff12,13. This is of particular note, given that concerns over residual pulmonary regurgitation have been expressed with regard to early SAPIEN valves18. Importantly, the observed haemodynamic improvements appeared to persist over the year following the procedure, which has also been reported by other studies, regardless of the valve type used11,16,19,20. Sustained reductions in regurgitation and pressure gradients across the tricuspid valve were also apparent. This effect is a result of right ventricular volume/pressure relief and has been previously reported as an additional benefit of both surgical pulmonary valve repair and PPVI11,21.
Over 84% of Pulmonic S3 patients were in NYHA Class I after the procedure (70% more than at baseline), with excellent functional improvement across the board. In comparison, only 63% of patients in the Pulmonic XT registry achieved NYHA Class I after PPVI (53% more than at baseline), despite a smaller proportion having been in NYHA Class III/IV prior to the procedure11. Furthermore, while 80% and 83% of adults and children, respectively, were reported to be in NYHA Class I following Melody valve implantation, 48% and 51% had already been in this class at baseline, representing increases of just 32% and 32%, respectively22,23.
SAFETY
Previously, PPVI has been associated with multiple procedural complications, including coronary compression, conduit rupture/tearing, stent or valve embolisation/dislocation, distal pulmonary artery damage, and access-site injuries24,25. In the present study, only two patients (3%) experienced any kind of complication, one of which was purely technical and both of which were fully resolved without further incident or need for conversion to open surgery. In comparison, an amalgamation of US prospective studies reported more frequent adverse events during PPVI using the Melody valve (9% of patients), including conduit rupture/tear (3%), access-site complications (2%), distal pulmonary artery perforation (1%), and coronary compression (1%)24. None of the last three events was documented in the present study, with conduit puncture occurring in only one patient (1%). Patients in the Pulmonic XT registry also experienced relatively more frequent technical complications, including device malfunction and valve dislocation requiring surgical correction (2.2% and 4.4%, respectively), with procedural failure in 6.5% of patients11. These events were also absent from the present study, perhaps reflecting use of the more accommodating distal flex catheter Commander delivery system, which features handles to measure the degree of articulation, a fine adjustment wheel and a balloon lock knob13; however, the possible effect of increased operator experience and better patient selection cannot be discounted. Strikingly, while 8.9% of patients in the Pulmonic XT registry experienced conductance disturbances requiring treatment11, no such arrhythmias were seen in the present study. The reason for this substantial difference remains unclear and merits further investigation. Nevertheless, it appears that PPVI with the S3 is an extremely safe procedure, with no procedural mortality, excessive blood loss or embolisation.
Over the longer term, stent fracture and endocarditis are the principal concerns. Neither event had occurred by one year in the present study. The former complication has probably been mitigated by extensive durability, hydrodynamic and fatigue testing of the S3 valve in combination with the now conventional practice of pre-stenting to prevent stent fracture8. Nevertheless, more recent Melody valve studies that employed pre-stenting report incidences of this adverse event22,24,26, with one trial reporting as many as 32% of patients being affected at six months15. Conversely, SAPIEN valves have consistently been associated with a lack of stent fracture16,20,27. Prior studies with the SAPIEN family of valves have also shown endocarditis to be rare or absent11,16, with data suggesting that it may be more common with Melody valves23,24,28. Nevertheless, the current data on longer-term adverse events following PPVI with the SAPIEN 3 are minimal; the ongoing COMPASSION S3 study will provide additional information as to the safety and effectiveness of the procedure29.
Study limitations
There were several limitations to the present study. Firstly, no minimum requirements as to the investigator and site experience with PPVI or the S3 valve were specified. As such, learning curve and operator experience may have affected outcomes. Secondly, retrospective acquisition of data was necessary for some patients, limiting the reliability and breadth of variables available. Furthermore, as an observational registry, questionnaire fields were filled based on site standard observations, and consequently missing data were not uncommon. Nevertheless, the flexible registry design allowed us to gather as much information as possible over a large range of patients, within a structured format. Indeed, while our sample size was modest compared to other registries, this was unavoidable given the current rarity of PPVI with the S3 valve. Finally, given the early stages of the registry, few data on outcomes at later time points were available. More information will be obtained as the study continues, with follow-up planned for up to two years.
Conclusions
PPVI with the S3 valve appears to be safe and feasible in patients with congenital heart defects, including those with native RVOT anatomy. The procedure results in good short-term haemodynamic and functional outcomes, with very few adverse events; however, the endurance of such positive safety and efficacy outcomes remains to be demonstrated over the longer term, as does device durability. Nevertheless, the present study paves the way for more liberal implantation of the S3 valve in the pulmonic position.
| Impact on daily practice PPVI with the S3 valve appears to be safe and feasible in patients with congenital heart defects, including those with native RVOT anatomy. The procedure results in good short-term haemodynamic and functional outcomes, with very few adverse events. The present study paves the way for more liberal implantation of the S3 valve in the pulmonic position. |
Acknowledgements
Data were captured using the s4trials Software (Software for Trials Europe GmbH, Berlin, Germany).
Funding
This work was supported with a research grant provided by Edwards Lifesciences, Nyon, Switzerland.
Conflict of interest statement
S. Hascoet, J. Bentham, R. Giacomo Carere, P. Ewert, E. Katarzyna Biernacka, O. Kretschmar, P. Bramlage and N. Haas have received research funding, consultancy and/or lecture honoraria from Edwards Lifesciences. J. Kurucova and M. Thoenes are employees of Edwards Lifesciences. The other authors have no conflicts of interest to declare.

