
Abstract
Aims: Conduit rupture is a serious complication encountered during percutaneous pulmonary valve implantation (PPVI). We sought to evaluate the incidence and predictors of conduit rupture during right ventricular outflow tract (RVOT) transcatheter treatment.
Methods and results: All consecutive patients who underwent transcatheter RVOT treatment from May 2008 to December 2011 were prospectively studied. Baseline demographics along with incidence, predictors and outcomes of conduit rupture with various transcatheter therapies were reviewed. Conduit rupture occurred in nine out of 99 patients (9.09%). All conduit ruptures occurred during balloon dilatation. Significant risk factors included heavy calcification (p<0.05, OR=16 [1.87-357]), and conduit type (homograft/others; p<0.05, OR=4.37 [1.1-17.8]). Other factors such as prolonged time interval between prior surgical RVOT repair and interventions, use of high-pressure balloons, balloon diameter, and overexpansion of conduit statistically failed to show any association. All patients were managed in the cardiac catheterisation laboratory. There were no delayed complications during a mean follow-up period of 2.3±0.95 years.
Conclusions: Conduit rupture is a serious complication. Heavy calcification and homograft conduit were significant predictors. Immediate diagnosis with the use of targeted interventional therapies should be attempted before proceeding with PPVI.
Abbreviations
Ao: aortic
BMS: bare metal stent
PA: pulmonary artery
PPVI: percutaneous pulmonary valve implantation
RA: right atrium
RV: right ventricle
RVEDV: right ventricular end-diastolic volume
RVOT: right ventricular outflow tract
Introduction
Transcatheter intervention for the management of dysfunctional RVOT has expanded over time to include simple balloon dilatation, bare metal/covered stent implantation and percutaneous pulmonary valve implantation (PPVI). While long-term complications from such interventions are dominated by structural alterations of the device (e.g., stent fractures, etc.), life-threatening periprocedural complications may sometimes compel urgent surgical repair1,2. The stepwise approach for RVOT transcatheter therapy usually includes careful pre-procedural patient selection, predilatation followed by pre-stenting with a bare metal stent, and finally valved stent implantation after adequate RVOT preparation. It is of paramount importance to evaluate carefully the angiographic appearance of the RVOT and its surrounding area, and to assess the expandable properties of the RVOT, its adherence to the sternum and the risk of coronary compression. The surgically implanted RVOT conduit has enabled correction of highly complex defects; however, it undergoes progressive degeneration with age3-6. Dystrophic calcification on such degenerated material may weaken the conduit and make it brittle. Every step of RVOT preparation and PPVI carries the risk of conduit rupture. Anecdotal case reports of conduit rupture have been described during transcatheter therapies for conduit failure7. With the widespread use of the percutaneous pulmonary valve, there are now some data on conduit-related complications noted during RVOT interventions and PPVI8,9. Bail-out surgery has been the mainstay of treatment for large tears with expanding extracardiac extension2. There is clearly a lack of data from multicentre studies on the possible risk factors for conduit rupture. We sought to investigate the incidence, predictors and outcomes of conduit rupture in the setting of transcatheter RVOT interventions from a large multicentre database.
Methods
SELECTION CRITERIA
This is a prospective, analytical, observational study enrolling all consecutive patients who underwent transcatheter RVOT therapies from May 2008 to December 2011 for RVOT obstruction, pulmonary regurgitation or mixed lesions. Inclusion criteria were as follows: 1) age >five years, 2) presence of a RVOT circumferential conduit or treated anteriorly with a BMS, and 3) indications to intervene for obstruction, PR or a combination of both. Indications to intervene were defined as followed: patients in NYHA Class I were considered candidates if the PR was severe (RVEDV >170 ml/m2 on MRI) and/or if RV systolic pressure was isosystemic or if the mean RVOT gradient was greater or equal to 40 mmHg on echocardiography. Patients in Class II were candidates if the PR was moderate to severe (RVEDV >140 ml/m2) and/or if RV systolic pressure was 3/4 systemic or if the mean RVOT gradient was greater than or equal to 35 mmHg on echocardiography. Patients in NYHA Class III and IV were candidates if the PR was moderate to severe (RVEDV >140 ml/m2) and/or if RV systolic pressure was 2/3 systemic, or if the mean RVOT gradient was greater or equal to 30 mmHg on echocardiography.
All included patients were potential candidates for PPVI and the spectrum of RVOT procedures included balloon dilatation, with or without bare metal stent implantation, with or without PPVI. Patients were prospectively enrolled in three different centres across France (Paris, Marseille and Bordeaux). Exclusion criteria included patients with native unoperated RVOT, native pulmonary valve stenosis, patients below five years of age and/or patients weighing less than 20 kg. The study protocol included assessment of demographic data, procedural details, complications, and immediate and midterm outcomes. Periprocedural and follow-up data were collected in a large institutional review board-approved database. With strict selection criteria and procedure protocol, the population cohort and the anatomical substrate thus remained uniform. The data were prospectively acquired with prior ethics committee and institutional review board approval.
PRE-PROCEDURE EVALUATION
All patients underwent a pre-inclusion clinical evaluation including surface electrocardiogram, echocardiography, chest X-ray, exercise testing and non-invasive imaging (MRI or CT scan). Most of the patients in this study underwent MRI (97%) to quantify RV volumes. CT scan was used only in those with contraindications for MRI due to implanted cardiac rhythm devices (n=3).
Cardiac catheterisation
Cardiac catheterisation was performed under general anaesthesia or deep sedation. The peak-to-peak RV to PA gradient was calculated as the difference in systolic pressure between RV body and the main PA on pullback. Patients were categorised according to primary transcatheter therapy indication based on the haemodynamic inclusion criteria met at the time of the enrolment. If the RV/Ao systolic pressure ratio and RVOT gradient were greater than 0.66 and 25 mmHg, respectively, then stenosis was deemed as the primary indication. If an angiographically significant pulmonary regurgitation was present and the RVOT gradient was lower than or equal to 25 mmHg, pulmonary regurgitation was deemed as the primary lesion. The others were categorised as mixed lesions. RVOT and aortic root angiograms were performed in two views (lateral and four chamber views) in all patients. Aortic angiography (global and/or selective) was performed to assess the proximity of the bare metal stent and/or Melody® transcatheter pulmonary valve (Medtronic, Minneapolis, MN, USA) landing zone to the coronary arteries. In patients with obstruction or mixed lesions, this proximity was assessed prior to and during balloon inflation (ATLAS®; Bard Peripheral Vascular, Inc., Tempe, AZ, USA; Mullins-X™ and TYSHAK®; NuMED, Inc., Hopkinton, NY, USA; Cristal; BALT, Montmorency, France). Balloon diameter was chosen equal to the targeted BMS or Melody diameter which in turn was chosen according to age, initial conduit diameter and conduit type (expandable or not expandable), and not to RVOT dimensions at the time of the procedure. For example, if we expected to implant a Melody using an 18 mm Ensemble® delivery catheter (Medtronic) then the ballooning was performed using an 18 mm balloon. If the coronary arteries were found proximal to the landing zone during this assessment, additional balloon inflation was performed using a larger balloon (diameter equal to the external diameter of the Melody). The type of balloon was selected by the operators and left to operator preference (not defined in the protocol). However, the aims of this balloon inflation were multiple and, for balloon selection, each operator kept in mind: 1) if the stenosis could be relieved (complete opening of the stenosis?); 2) if recoil appeared after deflation; and 3) the assessment of the proximity of coronary arteries.
Angiographic and haemodynamic assessment was repeated at each step to assess the efficacy of dilatation and the integrity of the conduit. The procedure was concluded at this stage: 1) if the results were deemed adequate; 2) in case of contraindications to stents (proximity to vulnerable tissues); 3) in case of inability to open a stenotic lesion despite of the use of adequate balloon catheters; or 4) when the PR was limited by angiographic grading. In children, correction of PR induced by BMS insertion or balloon dilatation was not indicated in patients (with no prior significant RV dilatation) with normal PA anatomy (i.e., no peripheral PA stenosis or PA hypoplasia) and without pulmonary hypertension. Because of this institutional policy, we expected to find more BMS implantation in children than in adults.
If the results were not adequate based on these criteria, patients underwent bare metal stent implantation (single or multiple based on lesion complexity) and/or Melody valve implantation. Following stent insertion, angiographic and haemodynamic assessment was repeated.
In selected patients, the Medtronic Melody® transcatheter pulmonary valve (Model PB10) was then inserted using the Medtronic Ensemble® transcatheter valve delivery system (NU10). Most of the patients had pre-stenting of the RVOT (CP Stent™; NuMed, Hopkinton, NY, USA, or ev3 Max™ LD; ev3 Endovascular, Inc., Plymouth, MN, USA). Patients with residual RVOT gradient over 25 mmHg underwent RVOT post-dilatation with a high-pressure balloon of an appropriate diameter (Mullins-X™; NuMED, and/or ATLAS®; Bard) with the objective of minimising the gradient. Parenteral heparin and antibiotic prophylaxis were administered per institutional protocol and patients were discharged on aspirin 100 mg oral daily.
In patients with mixed or obstructive lesions, success of the procedure (balloon, BMS implantation or Melody) was defined as a drop in the peak systolic RV-PA pressure gradient by more than 50% and a reduction in RV/aortic systolic pressure ratio to less than 0.50. Elsewhere, success was defined as correction of PR.
Follow-up
All patients were scheduled for outpatient clinic visits at one, three, six, 12, and 24 months following the transcatheter procedure. A repeat transthoracic echocardiogram along with detailed clinical evaluation was performed at every follow-up.
Features of conduit rupture
In order to characterise RVOT characteristics including calcification, extent of balloon expansion, and conduit rupture, all angiographic data were reviewed by two authors blinded to the outcomes. RVOT calcification on cineangiography was classified as non-visible calcification or specks of calcification (none or minimally calcified, grade 0), and moderate to severe, thin linear to thick circumferential calcification (moderately to severely calcified, grade 1). Conduit ruptures were classified as absent (grade 0), minimal rupture without extension (grade 1), moderate rupture with extension but contained and without haemodynamic instability (grade 2), and severe rupture with extension causing haemothorax or haemopericardium and with haemodynamic instability requiring blood transfusion (grade 3). The types and sizes of the balloon were collected and analysed. The ratio between balloon diameter and conduit diameter was calculated. Oversizing was defined as a ratio of >1, or >100%.
Statistical analysis
PASW statistics 17.0 (formerly SPSS Statistics) (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Nominal variables are expressed as numbers and percentages and compared by Fisher’s exact test or χ2 test when appropriate. Ordinal variables are presented as mean±standard deviation and compared with the Wilcoxon rank-sum test. Continuous variables are expressed as mean±standard deviation and compared with the independent variables t-test. Patients were stratified based on the presence (rupture +: grade 1 and above) or absence of conduit rupture (rupture –: grade 0). All tests were two-sided and a p-value of <0.05 was considered statistically significant. Multivariable logistic regression models were constructed using selected combinations of variables significant to p<0.05 on univariate analysis. Intra- and inter-observer variability was assessed. The degree of agreement was quantified by kappa. When the grading was ordered, the calculation was made with weighted kappa to assess the quality of the ordering. Inter-observer variability was calculated testing all patients enrolled in the study. Intra-observer variability was calculated testing a sample of 50 patients randomly selected for calcification grading and in all patients in the subgroup of conduit rupture for severity grading. Subjects categorised differently were reviewed by the two observers and a consensus between observers was reached for “final” grading. Statistical analysis was performed using the grading given identically by the observers, or the consensual and final grading given by the observers in case of disagreement.
Results
DEMOGRAPHICS
Ninety-nine patients met the inclusion criteria over the period. Forty-three patients were children (mean age 13.36 years, range five to 17.88 years). Fifty-six patients were adults (mean age 27.16 years, range 18 to 54.82 years). Seventy-two patients were treated in Centre A (30 children and 42 adults), six in Centre B (two children and four adults) and 21 in Centre C (11 children and 10 adults). Pre-procedural mean systolic RV pressure, mean systolic RV to PA gradient and ratio between systolic RV and aortic pressures were respectively: 73±19.7 mmHg (25-130), 49±21.5 mmHg (5-115) and 1±0.23 (0.19-1.44).
Eighty-three patients out of a total of 99 included in the study received a Melody valve (83.9%). Distribution among centres is provided in Table 1. The remaining patients (n=16; balloon dilatation alone in five; bare metal stent implantation in 11) did not undergo Melody valve implantation because: simple balloon dilatation resulted in good haemodynamic results without creation of significant PR (n=4); proximity of coronary artery (n=1); stent implantation preserved conduit valve function (n=2); and BMS implantation alone resulted in good haemodynamic improvement in the rest (n=9). All patients who received a BMS alone were children from Centre A (p=0.0001 [BMS vs. Melody]). This statistical difference was expected and is related to institutional policy. Five children had conduits smaller than or equal to 14 mm in diameter with two being non-dilatable. Four out of five were treated with a BMS alone (two expanding the initial conduit diameter) and one received a Melody valve. No adult had a conduit smaller than 16 mm in diameter.
In patients with an obstructive lesion with or without PR, balloon dilatation was performed using a high-pressure balloon in 22, medium-pressure balloon in 50 and low-pressure balloon in four. The type of balloon was not different when comparing age group (p=0.92). The type of balloon was statistically different between centres (p=0.0084). Centre A used more high-pressure balloons (37.7% of the total) than Centre C which used medium-pressure balloons almost exclusively (94.4%). Balloon/conduit diameter ratio was not different between centres for the total population, or adult subgroups, but Centre A used larger balloons than Centre C in the child population (p=0.0334).
CONDUIT RUPTURE
Conduit rupture was noted in nine patients (9.09%: four children and five adults) during RVOT interventions (Table 2). Rupture occurred after balloon dilatation in all patients. Angiographically obvious and severe conduit rupture with extension causing haemodynamic instability was noted in only two patients (grade 3, 22.22%: 2% of the total study population). For the rest, rupture was diagnosed during stepwise angiographic assessment. The site of rupture was proximal in one, in the middle of the conduit in six and distal in two (at the PA bifurcation in one): rupture was identified anteriorly (n=5) and posteriorly (n=4). The site of opening of the balloon (release of balloon indentation) during its full inflation retrospectively precisely predicted the site of conduit rupture in all patients. The intra- and inter-observer variability for conduit rupture grading was low. The agreement between observers was excellent, with weighted kappa score at 0.969 (inter-observer variability) and 0.967 (intra-observer variability), respectively.
Rupture was classified as grade 1 (minimal) in two, grade 2 (moderate and contained) in five and grade 3 with haemothorax and haemodynamic instability in two (Figure 1-Figure 4).
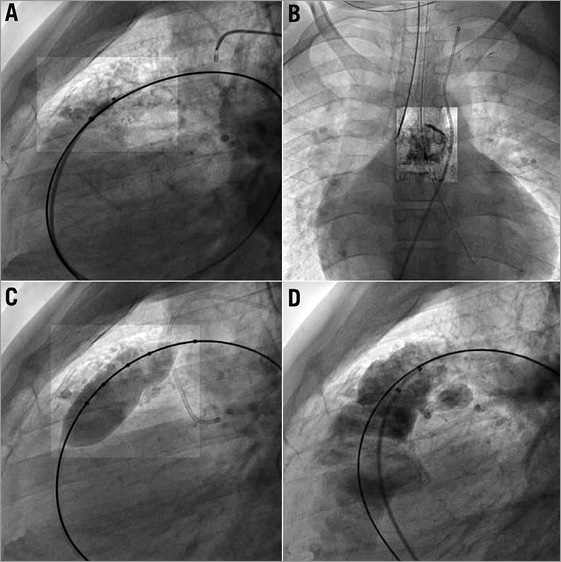
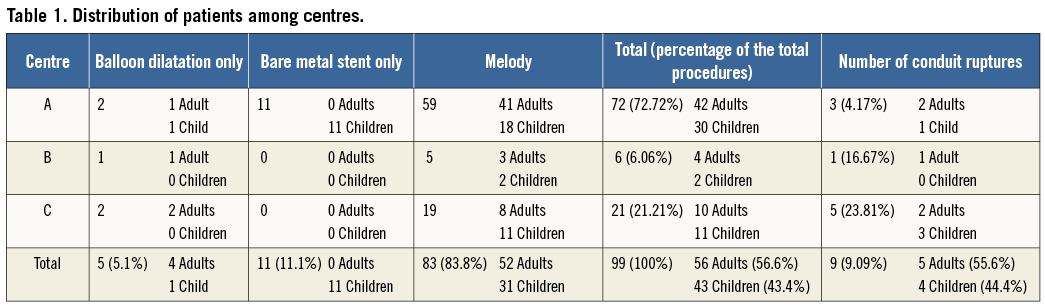
Figure 1. Cineangiogram still frame from patient 7 showing a heavily calcified 23 mm homograft (A & B). A posterior indentation on a 20 mm Mullins balloon is seen on panel C. Moderate, posterior RVOT rupture occurred after balloon inflation (D). The rupture is contained.
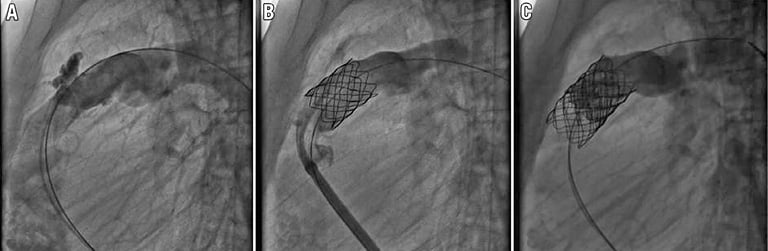
Figure 2. Cineangiogram still frame from patient 6 showing a moderate, anterior and contained conduit rupture (A). A CP covered stent is inserted to seal the rupture (B). Angiogram after Melody valve implantation showing disappearance of pulmonary regurgitation (C).
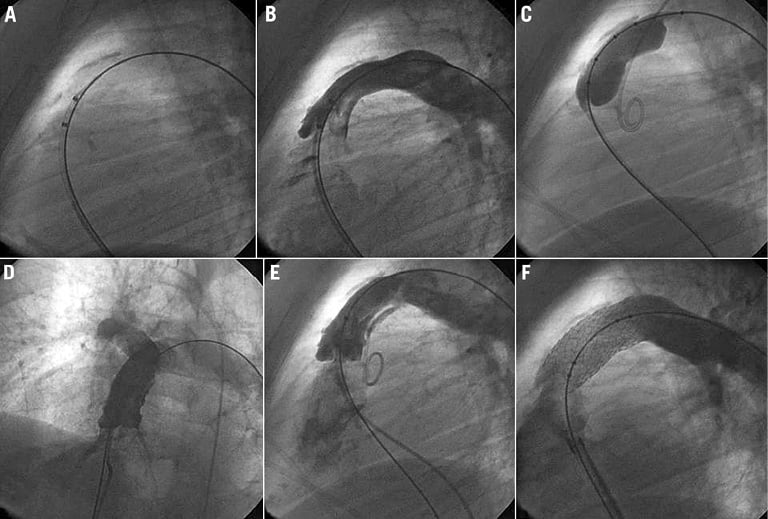
Figure 3. Cineangiogram still frames from patient 8. A) & B) The conduit is moderately calcified all along the conduit. C) Balloon inflation of an 18 mm Mullins balloon showing posterior indentation at the mid part of the conduit. D) Angiogram in a four-chamber view showing a contained rupture. E) Cineangiogram still frame showing posterior, contained conduit rupture. F) Angiogram in lateral view after placement of a non-covered bare metal stent showing the disappearance of the dissection.
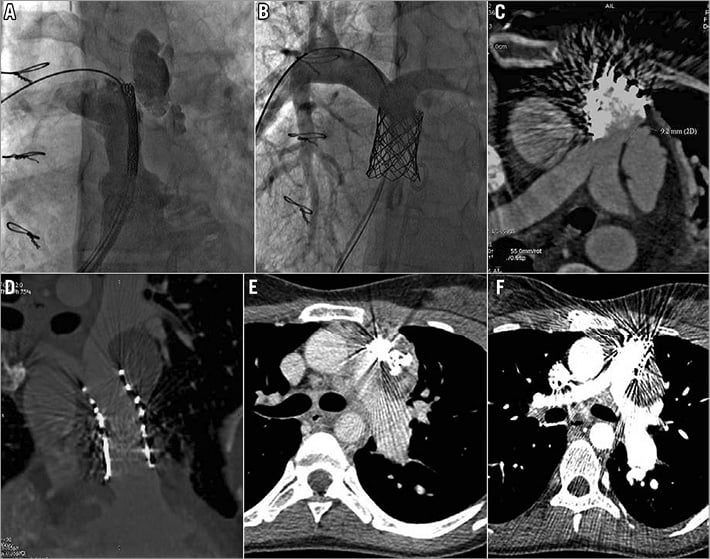
Figure 4. Cineangiogram still frames from patient 3 (homograft) showing anterior, severe conduit rupture before (A), and after covered stent implantation (B). A CT scan was performed the next day due to expanding haemothorax. C) The covered stent did not cover the site of rupture that was at the distal end of the conduit but proximal to the PA bifurcation. E) A PDA device occluder was deployed at the mouth of rupture with no benefit. D) & F) A short covered stent (CP Stent™ [CP8Z22]; NuMED) was then carefully positioned distally to seal effectively the rupture without impinging on the newly implanted Melody valve.
No patient required urgent or delayed surgery for conduit rupture. The rupture was treated percutaneously in seven out of nine patients, and carefully observed with no specific treatment for the rest. The therapy was immediate on diagnosis and prior to valve insertion in most (6/7, 85.7%). The rupture was “sealed” with implantation of a bare metal stent in three patients (flap lesions), with a balloon-expandable covered stent in three, and with a PDA device plus a balloon-expandable covered stent in the last one (delayed treatment).
One patient had delayed definitive treatment of the rupture. This patient had significant conduit rupture and we used a Melody valve as a covered stent to seal the rupture. There was significant angiographic resolution with no extension after Melody valve implantation and the patient left the cardiac catheterisation laboratory with no other specific management for rupture. This patient required re-catheterisation the following day due to expanding haemothorax. Our assumption that the covering of the Melody valve would seal the rupture proved wrong. The procedure was then quite challenging as the site of rupture was at the distal end of the conduit but proximal to the PA bifurcation, making intervention tricky due to the newly implanted Melody valve. A PDA AMPLATZER™ duct occluder (AGA/St. Jude, St. Paul, MN, USA) was deployed at the mouth of the rupture with no benefit. A short covered stent (CP Stent™ [CP8Z22]; NuMED) was then carefully positioned such that it would not impinge on the valve function proximally or jail the pulmonary arteries distally. The rupture was thus effectively sealed. The patient was discharged two days after the procedure.
In two patients with grade 1 rupture (minimal), since there was no significant extension or expansion of the rupture after two repeat angiograms at 15-minute intervals, it was decided to continue conservative management. The final angiograms in both patients showed completely sealed perforation. The first patient, who had complete relief of obstruction after balloon dilatation, did not undergo BMS or valve implantation. For the second patient, a BMS was deployed after complete sealing of the rupture because of an incomplete haemodynamic result after balloon inflation alone. This BMS implantation may possibly have prevented delayed extension of the rupture. Both were discharged home without early or late complications.
Haemodynamic results after endovascular treatment in patients with or without conduit rupture were comparable. There was no periprocedural or hospital mortality related or unrelated to conduit rupture. No patient underwent RVOT intervention after discharge up until the last follow-up related to conduit rupture. After a mean follow-up of 2.3±0.95 years, all patients showed good clinical improvement in functional class and good haemodynamic findings on transthoracic echocardiography.
Risk factors
RELATIONSHIP WITH SUBSTRATES
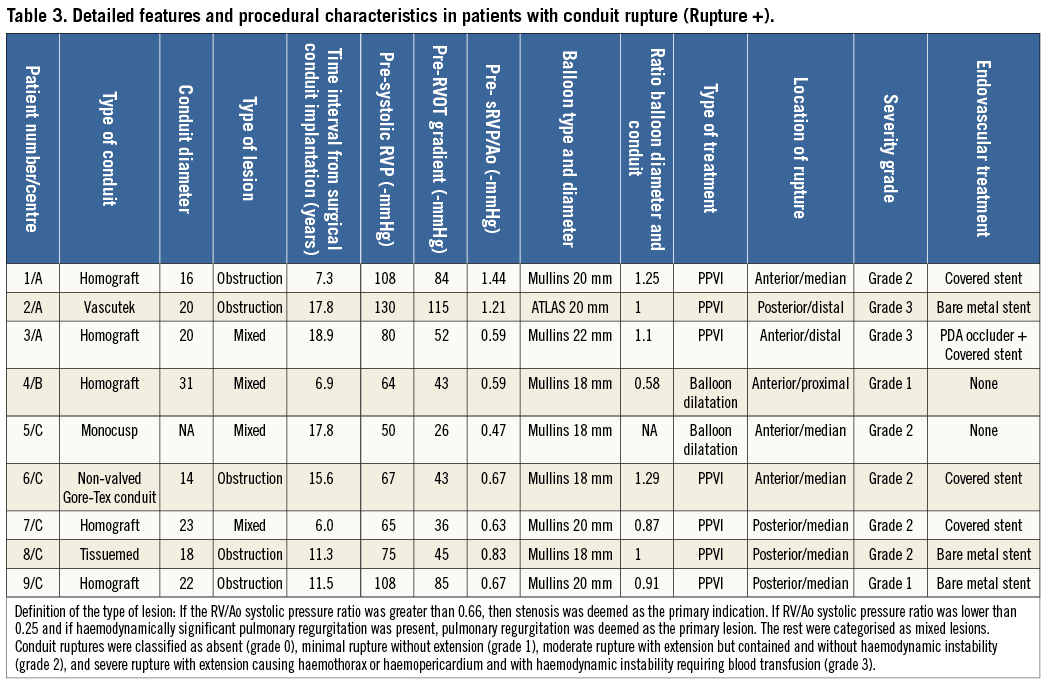
Patients with conduit rupture had an initial surgical conduit placed 12.6±5.1 years (range: 6-18.9 years) prior to transcatheter intervention. This did not differ statistically from those without this complication (p=0.85). Rupture was noted in five out of 25 patients with homograft, and four out of 74 with conduits or patched RVOT (p=0.0396; OR 4.37 [1.1-17.8]). There was no correlation with baseline conduit diameter (Rupture [-]: 19.5±3.4 mm vs. Rupture [+]: 20.5±5.2 mm; p=0.47).
RELATIONSHIP WITH RVOT DISEASE
Rupture occurred exclusively in patients with obstructive or mixed lesions. However, there was no statistical difference when comparing the pre-procedural haemodynamic data between patients with and without conduit rupture (systolic RVP: p=0.12; RVOT gradient: p=0.17; systolic RVP/AoP: p=0.95) in the total population as well as in patients with obstruction or mixed lesions only. Patients with conduit rupture had minimal RVOT calcification in one (grade 0, patient 5) and moderate to severe in the remaining (grade 1). The degree of calcification of RVOT was statistically higher in patients with conduit rupture as compared with patients without conduit rupture (p=0.001; OR 16 [1.9 - 357]; RR 12.9 [1.7-98.7]). The inter- and intra-observer agreements regarding the degree of calcification were excellent with kappa scores at 0.979 (inter-observer), 0.959 for observer 1 and 0.918 for observer 2 (intra-observer).
RELATIONSHIP WITH TRANSCATHETER PROCEDURES AND CENTRES
Contrary to what one might have expected, conduit rupture did not occur commonly in patients where balloon dilatations were performed using high-pressure balloons (p>0.5), nor when balloon diameter was greater than 100 or 120% of the initial conduit diameter (Table 3). Balloon diameters were comparable between the two groups. Frequency of occurrence was different between centres with a lower occurrence in Centre A and a higher incidence in Centre C (Centre A: 3/72=4.2%; Centre B: 1/6=16.7%; Centre C: 5/21=23.9%; A vs. C, p=0.017. However, conduit type was also different. The proportion of treated homografts was different in the three centres (p=0.00043, Centre A: 11/72, Centre B: 2/6 and C: 12/21). Moreover, the severity of the conduit rupture was greater in Centre A, with two out of three ruptures being grade 3 compared with 0 out of five in Centre C.
Multivariate analysis
Multivariable logistic regression models were constructed using selected combinations of variables significant to p<0.05 on univariate analysis (i.e., degree of calcification, centres, and homograft). The only risks factors found statistically significant were: high degree of calcification (p=0.0081, HR=20.9 [2.2-198]), and patients treated in Centre C (p=0.0214, HR=8.2 [1.3-49.6]). Homograft did not reach statistical significance (p=0.5355).
Discussion
PPVI is now a widely accepted treatment for the management of dysfunctional, repaired RVOT10-12. The preparation of RVOT for PPVI may cause conduit-related complications, and conduit rupture remains an uncommon but serious and potentially life-threatening complication. It was believed that preparation of RVOT using aggressive balloon dilatation and pre-stenting may cause conduit rupture; however, the precise incidence and risk factors are not well reported13-15. Peng et al reported minimal extravasation of contrast in six of 221 patients undergoing endovascular right ventricular outflow tract stenting. None developed haemothorax or haemopericardium; one required a covered stent and the rest were all managed conservatively9. Kostolny et al reported emergency surgery in two patients with conduit rupture out of 152 patients who underwent PPVI2. In contrast to prior studies we have a large series of patients with an in-depth analysis of the possible predictors for conduit rupture.
PPVI is a technically challenging procedure with an estimated procedural complication rate as high as 15%, which may include stent fracture, coronary compression and conduit rupture16. Conduit rupture occurred in nine patients (9.09%) in our series; this was noted exclusively in patients with RVOT obstruction with or without PR as primary lesion. Interestingly, rupture occurred during RVOT balloon inflation performed to test the expandability of the RVOT and the proximity of coronary arteries. We believed that a higher than the reported incidence in our study was related to a more systematic report and search of the ruptures. Indeed, power dye injections were performed at each step of the procedure specifically to look for any conduit rupture and that is not a common practice among operators. Lesions were limited and diagnosed by angiograms only in the vast majority of patients7,9. Lesions led to a clinically relevant rupture in only two patients (2%). This rate is very similar to what has previously been reported by others8. We treated the vast majority of ruptures percutaneously (clinically relevant or not). It is, however, unknown if a rupture evolves from one grade to another.
We believe that, in patients with coronary arteries close to the Melody/BMS landing zone, it is important to obtain full balloon expansion to exclude the possibility of potential coronary compression after stent deployment (bare or valved stents). It is also important not to use undersized balloons or limit balloon inflation, as this may prove ineffective to predict coronary compression. In patients with stenosis or mixed lesions, the usual practice is to limit the aggressiveness of balloon testing using a 16 mm or smaller balloon diameter with the idea of preventing conduit rupture. This assumption is not supported by our findings. The choice and diameter of balloon had no impact on the incidence of complications. Moreover, overexpansion up to 1.5, highlighted in our series, did not increase the risk of rupture. However, most relevant ruptures occurred in the centre testing the RVOT more aggressively (Centre A).
The only risk factors for conduit rupture we found were related to the RVOT substrates. Conduit rupture occurred with a higher rate on univariate analysis in patients with calcified homografts. A more careful approach (smaller/low-pressure balloon favoured over direct stenting with a covered stent) might be useful in this subset of patients, but this approach remains to be proven.
In our experience, all ruptures occurred at the site of the opening of maximum balloon waist, which we have noticed to be the commonest site of balloon and, vis-à-vis, conduit rupture. All ruptures, including complex anastomotic, proximal or distal ruptures, could be treated using transcatheter techniques. Careful evaluation is mandatory to understand precisely the site, the type and the extent of rupture. Recording of balloon expansion and site of balloon opening is recommended since it predicted the site of rupture in our population. However, extension of the rupture could not be predicted based only on this feature.
An uncovered stent can be used to treat a flap but most of the time covered stents are required. In our opinion, treatment should be performed prior to Melody valve implantation; it is important to note that the Melody valve should not be considered or used as a covered stent for the management of conduit rupture. Once the Melody is implanted, the working space for further interventions is considerably reduced and any further instrumentation has the potential to damage the newly implanted valve leaflets. However, if the rupture extends after Melody valve implantation, possibly one may cover the Melody valve, and another Melody valve may then be implanted once the rupture is sealed7. Other occlusive devices such as the AMPLATZER occluder (St. Jude Medical) could be used at the anastomotic rupture site. If that too fails, then salvage surgery may be the only option. None of our patients required emergency or delayed surgery for conduit rupture.
The difference in outcomes among the three centres on the incidence of RVOT rupture certainly cannot be ignored since this factor was significant by univariate and multivariate analysis. However, we found no difference when comparing lesion treatment (conduit/balloon diameter ratio), type of lesions, and haemodynamic data. Centre A, which had the lowest incidence of rupture, was the most aggressive in RVOT testing with the most frequent use of high-pressure balloons. Y. Boudjemline proctored the other two centres participating in the study. Treatment of all patients from Centre B was performed by J-B. Thambo, with the help of Y. Boudjemline. The first seven patients from Centre C were treated in a similar manner while being proctored by A. Fraisse. All 14 subsequent patients were treated according to a defined protocol thus reducing the operator bias. The only factor we found different was the higher frequency of homografts in Centre C. However, this did not reach statistical significance on multivariate analysis.
Limitations
Calcification grading was found to be an important predictor of conduit rupture. Despite excellent reproducibility between observers, our qualitative scoring by angiography does not provide a precise quantification of calcification. Other imaging tools, in particular CT scan, certainly provide more accurate quantification (Agatston score) of calcifications by precisely measuring the Hounsfield Unit in the area of interest. We were unable to correlate our qualitative scoring with a more quantitative one. Beside calcification information, a CT scan is also interesting for coronary assessment before RVOT intervention. Keeping in mind the radiation exposure, one should consider performing a CT scan in that specific population.
Lack of randomisation may be seen as one of the limitations; however, since conduit rupture is a rare complication, it may be hard to design a prospective randomised controlled trial in a larger series of patients. One of the minor limitations of the study is the rare lack of documentation from surgical charts (n=4), with absence of conduit diameter details. However, since this is only in a minority of patients, we believe that it should not have a major impact on the analysis. Multivariate analysis usually requires at least 10 events per factor studied. The low number of events might explain why we were unable to find more significant factors. Multi-institutional series, including a larger number of patients, might be interesting to find more predictive factors.
Conclusions
Conduit rupture is a serious complication noted during transcatheter therapy of dysfunctional RVOT in patients with prior surgical repair. Heavy calcification, homograft conduit, and centre were significant predictors for conduit rupture. Though most of the ruptures were contained, it is important to diagnose in a timely fashion and use appropriate and targeted interventional therapies using a bare metal stent and/or covered stents to prevent expansion and extension of the rupture before proceeding with PPVI.
| Impact on daily practice Conduit rupture is a serious complication occurring during balloon dilatation performed prior to transcatheter pulmonary valve implantation. Despite this risk, we recommend aggressive testing of the proximity of coronary compression since the risk balance is in favour of conduit rupture. However, more attention should be paid when dealing with patients with heavily calcified homografts, this subset of patients being at higher risk for conduit rupture. Conduit rupture should be searched for carefully by power dye injection after each balloon dilatation. Precise understanding of location and extension of the rupture is important prior to endovascular treatment. Covered or uncovered stents should be placed before considering Melody valve implantation. |
Acknowledgements
We would like to acknowledge the help of the French Ministry of Health and URC HEGP.
Funding
This research benefited from a grant from the French Ministry of Health, STIC 2008.
Conflict of interest statement
Y. Boudjemline acts as a proctor for Medtronic. The other authors have no conflicts of interest to declare.

