
Abstract
Aims: Nowadays, transcatheter approaches are the treatment of choice for several congenital heart defects. However, adverse events may occur during interventional procedures. Even if the complication rate has been reduced remarkably because of learning curve and technological improvements, catastrophic events are still possible. The aim of this study was to review cardiac catheter complications that required surgical treatment during or after a percutaneous procedure.
Methods and results: We evaluated retrospectively a thirteen-year experience at our centre. We examined all transcatheter procedures involving device release or implantation needing surgical rescue. We performed 3,205 interventional catheterisation procedures with device release or implantation: ASD device closure (n=2,205), PDA device occlusion (n=355), VSD device closure (n=218), aortic coarctation or recoarctation stenting (n=199), pulmonary artery stenting (n=154) and pulmonary valve implantation (n=74). Complications that required surgical treatment occurred in 1.2% of cases. Early surgery was performed in 22 cases, while in 18 patients a surgical treatment related to late complications was performed in a mean follow-up of 17 months. There were no deaths in either group.
Conclusions: A spectrum of CHD can be treated today by transcatheter interventional procedures with good results and a low, but not negligible, risk of complications that require a surgical operation. The risk of developing late complications makes a long-term follow-up mandatory in such patients.
Abbreviations
ASD: atrial septal defect
CAC: coronary artery compression
CHD: congenital heart disease
ECHSA: European Congenital Heart Surgeons Association
PDA: patent ductus arteriosus
REV procedure: réparation à l’étage ventriculaire
RVOT: right ventricular outflow tract
TPVI: transcatheter pulmonary valve implantation
VSD: ventricular septal defect
Introduction
Cardiac catheter interventional procedures for congenital heart disease (CHD) are widely used and are the treatment of choice for several pathologies. Although the incidence of adverse events resulting from paediatric cardiac catheterisation has decreased remarkably over time, it still represents a major drawback1-4. In fact, complications may occur in paediatric patients during any procedural step due to various variables including CHD complexity, patient size, vascular access size and device technical limitations. However, more recently, appropriate patient selection, accurate pre-catheterisation assessment and the availability of smaller devices have reduced these risks1,2. On the other hand, in the last decade, the spectrum of treated CHDs has become wider and more complex, causing new types of complications that may need surgical treatment1-9.
On the basis of this, the aim of our study was to review the early and late cardiac catheter complications occurring in our unit and which required surgical treatment, with specific focus on the type of complication and the surgical procedures performed.
Material and methods
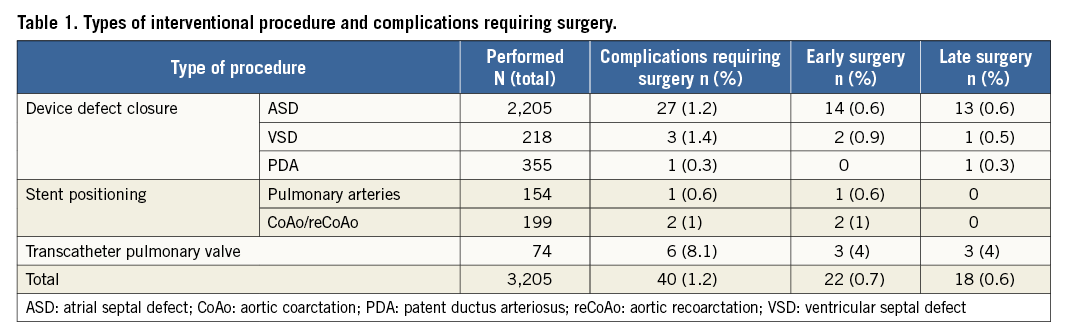
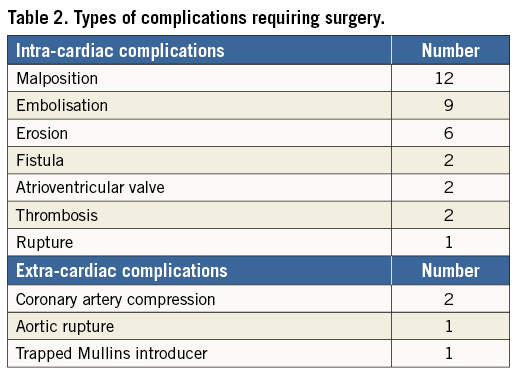
A retrospective analysis was conducted on all cardiac catheterisation procedures for CHD performed in our unit, from January 2000 to December 2013, in which a device was implanted and/or released and a complication occurred which required surgical treatment. The study was approved by the scientific ethics committee of our hospital. Patients included in the study were selected from our cardiac catheter and surgical databases. Data were collected from procedural reports and medical records. We included the following cardiac catheterisation procedures, where a device was either released or implanted: atrial septal defect (ASD) closure, ventricular septal defect (VSD) closure, patent ductus arteriosus (PDA) closure or stenting, aortic coarctation stenting, pulmonary artery stenosis stenting, pulmonary valve implantation (Table 1). Cardiac catheter procedure complications were classified as either intracardiac or extracardiac complications (Table 2). Furthermore, based on the time of occurrence, adverse events were divided into two groups: Group I, early complications (either rescue surgical procedures in the catheterisation laboratory or within the same hospitalisation), and Group II, late complications (surgery after hospital discharge during follow-up). Continuous variables are summarised as median and range, while categorical and ordinal variables are presented as frequencies and percentages.
Results
A total of 6,853 cardiac catheterisations were performed in our institution between January 2000 and December 2013. Of these, 3,205 interventional catheterisation procedures were for either device release or implantation. The following procedures were performed: ASD device closure (69%, n=2,205), PDA device occlusion (11%, n=355), VSD device closure (6.8%, n=218), aortic coarctation or recoarctation stenting (6.2%, n=199), pulmonary artery stenting (4.8%, n=154) and pulmonary valve implantation (2.3%, n=74).
Complications requiring surgical treatment occurred in 40 patients (1.2%); this formed our study group. Complications are listed in Table 3. Pulmonary valve implantation led to the highest complication rate (n=6, 8.1%), requiring open heart surgery. However, three of these six patients also underwent a late surgery for infective endocarditis.
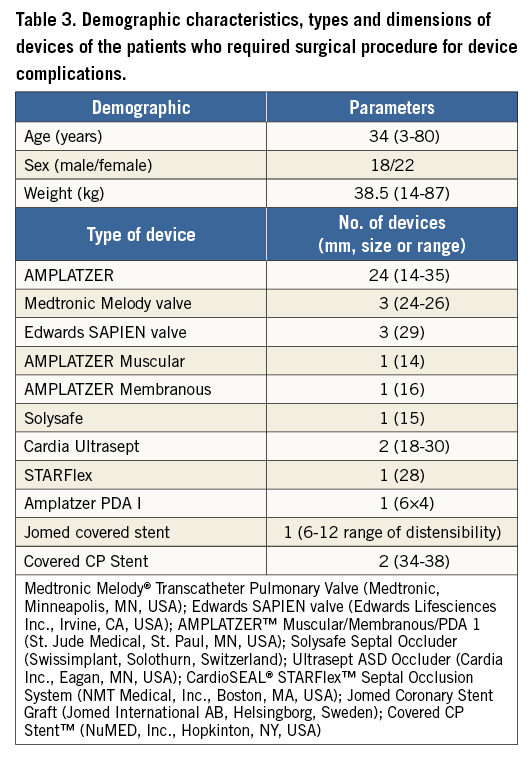
Rescue or early surgery was performed in 22 cases (55%), while in the remaining 18 (45%) it was required to treat late complications. There were no deaths in either group following the required surgical procedures, and all patients were discharged home after a median hospitalisation of seven days (IQR: six to 10 days). Demographic characteristics of the surgical groups, types and dimensions of the devices are shown in Table 3.
Group I: early complications
ASD DEVICE CLOSURE
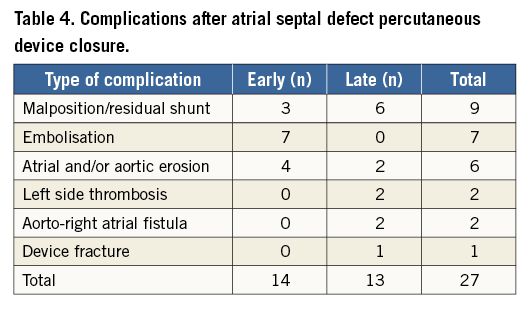
Percutaneous ASD closure was the procedure that most frequently required early or rescue surgery (14 out of 27 cases). Early surgery was required after device embolisation in seven patients (three on the day of the procedure and four the day after), erosion in four patients (two on the first day after the procedure, one after four days and one after seven days) or residual shunt/malposition in three patients (two on the day of the procedure and one on the day after the procedure). The other types of complication are listed in Table 4. In all cases, the device was removed and the ASD was closed with a heterologous pericardial patch. Four patients had erosion involving and damaging other cardiac structures: two cases of erosion of the left atrial wall, one case of damaging of the mitral valve and one case of erosion of the aortic wall. The postoperative course was uneventful in all patients.
VSD DEVICE CLOSURE
Two patients required surgery after percutaneous VSD closure. The first was a five-year-old male patient with a muscular VSD. After device positioning (AMPLATZER™ Muscular VSD Occluder, 16 mm; St. Jude Medical, St. Paul, MN, USA), the patient showed a significant residual shunting and he was operated on the same day. The device was removed and VSD closure was performed with a heterologous pericardial patch. The second patient was a seven-year-old female with a perimembranous VSD. After transcatheter closure of the defect, using a 14 mm AMPLATZER™ Membranous VSD Occluder (St. Jude Medical), she developed a significant tricuspid valve regurgitation which was caused by the entrapment of the septal leaflet into the device network. She was referred for immediate surgical treatment with device removal, tricuspid valve repair and VSD closure with an autologous pericardial patch.
PULMONARY VALVE IMPLANTATION
Among the 74 transcatheter pulmonary valve implantation (TPVI) procedures, three patients experienced early complications: two were cases of left coronary artery compression, and one was a right pulmonary artery occlusion due to device migration. The first patient had a history of Ross procedure, which had been performed twenty years earlier, while the second one had a REV procedure (réparation à l’étage ventriculaire), performed 28 years before. They had both undergone a previous attempt at valve implantation, one with a Melody® transcatheter pulmonary valve (Medtronic, Minneapolis, MN, USA) dilated up to 22 mm, and the other with a 26 mm Edwards SAPIEN valve, (Edwards Lifesciences Inc., Irvine, CA, USA). Both subjects showed left coronary compression. The implanted transcatheter pulmonary valves were removed, and the right ventricular outflow tract (RVOT) was surgically replaced by a pulmonary homograft. The third patient suffered a tetralogy of Fallot, which had been treated 40 years earlier with a VSD closure and transannular patch, and also had a 26 mm Edwards SAPIEN valve implanted. The device migrated into the right pulmonary artery, and the patient was operated for removal of the stent and surgical implantation of a stented bioprosthesis.
All three patients were operated on the same day as the percutaneous procedure, and they did not experience any further complications. They were discharged from the hospital in good condition.
AORTIC COARCTATION AND PULMONARY ARTERY STENTING GROUPS
Among 199 patients who underwent percutaneous treatment of aortic coarctation, there were two cases (1%) which required early surgical treatment.
One patient, a 45-year-old male with a history of end-to-end aortic repair at a neonatal age for aortic coarctation had an aortic rupture after stent implantation with left haemothorax. He was urgently transferred to the operating room. The stent was removed through a left thoracotomy approach and, after a femoro-femoral cardiopulmonary bypass, an 18 mm vascular Dacron prosthesis (Vascutek® Vascular Graft; Terumo Corp., Tokyo, Japan) was implanted between the aortic arch and the descending aorta. The second patient was a seven-year-old girl, operated on for aortic coarctation repair two years before, who experienced entrapment of a 12 Fr Mullins introducer sheath (Cook Medical, Bloomington, IN, USA) at the end of the aortic stent implantation. This complication required a laparotomy with longitudinal aortotomy to remove the introducer.
In the group of patients undergoing pulmonary artery stenting, we had just one case of stent embolisation. The device was found peripherally in the left pulmonary artery. After surgical stent removal, the artery was repaired with a pericardial patch.
Group II: late reoperations
Eighteen patients (18/40, 45%) underwent surgical treatment for complications linked to the interventional procedure from seven days to four years after device implantation (mean 17±8 months).
The majority of late complications occurred after ASD device closure (13/18, 72%). The indications for surgical intervention were: residual significant shunt in six patients (from two to 42 months after the procedure), atrial wall erosion in two patients (13 and 19 months after the procedure), aortic non-coronary sinus to right atrial fistula caused by device erosion in two patients (after seven and eight months), left side thrombosis, on the device site, in two patients after AMPLATZER ASD Occluder (St. Jude Medical) implantation (after two and 31 months), and device wire fracture (Solysafe Septal Occluder; Swissimplant®, Solothurn, Switzerland) in one patient after 36 months. In all patients the device was surgically removed and the atrial septal defect closed with a pericardial patch (Table 4).
In the remaining five patients, late complications included: significant residual shunting after VSD device closure in one patient, PDA occluder device migration into the descending aorta in one patient, and infective endocarditis of the transcatheter pulmonary valve in three cases. The patient with residual shunt was a female, 65 years old, operated on in infancy for tetralogy of Fallot. Twenty-four years after the first operation for interventricular residual shunt, a 16 mm AMPLATZER Muscular device (St. Jude Medical) was implanted. After 43 months, the patient presented a significant residual shunt. She underwent a surgical device removal and VSD closure with a heterologous pericardial patch.
The PDA occluder, which migrated into the aorta 13 months after the initial implantation, was retrieved through a left thoracotomy, and the PDA was ligated. Finally, the three patients who developed subsequent infective endocarditis underwent surgical pulmonary valve replacement after three, five, and 11 months for TPVI. The devices involved were a Melody valve dilated up to 24 mm, a 26 mm Edwards SAPIEN valve, and a Melody valve explanted up to 22 mm, respectively.
Discussion
In the past decade, the dramatic improvement in the field of interventional cardiology has led to a change in the strategy for managing many pathologies which in the past were considered only suitable for surgery. Indeed, this evolution represents a great advantage for these patients, as they can now benefit from less invasive treatments. The development of technologies has allowed us to perform more challenging procedures on smaller and younger patients2. This could be thought a major risk factor for rescue surgery after catheterisation.
In this study, we retrospectively analysed the large series of our interventional cardiology unit procedures in order to evaluate whether the risk of rescue surgery is significant, even in a high-volume centre such as San Donato. In our cohort, the highest number of adverse events was subsequent to ASD closure (the most frequent procedure), and percutaneous pulmonary valve implantation.
ASD transcatheter closure is rarely accompanied by early or late complications7,10. Among these, there are reports of recurrent cerebral embolism, cardiac perforation leading to tamponade, device malposition or embolisation in 4% to 20%, residual shunt in up to 30%, vascular trauma, thrombus formation on the device, or induced mitral regurgitation11-13.
In the population studied, the total rate of complications which required surgical treatment after percutaneous ASD closure was 1.2%; only 0.6% concerned early reoperations. There were 14 cases of early surgical operation after ASD device release. The most frequent cause was embolisation of the device followed by residual shunt and by erosion of the atrial wall with pericardial tamponade. In our group, the mortality rate was zero and the mean hospital stay was similar to that already published by Butera et al in relation to elective surgical treatment13. In the multicentre survey by the European Congenital Heart Surgeons Association (ECHSA), Sarris and associates showed data related to the rate of surgery after transcutaneous ASD closure1. In that study, it was shown that surgical complications can occur irrespective of the device dimensions. Moreover, even after closure of minor ASDs using small devices, adverse events may happen: serious complications have been reported for all types and sizes of device. This finding contradicts the loosely held and unfounded concept that small ASDs and small devices are risk free. However, the study lacks a statistical analysis on the potential risk factors for surgery. In addition, the limited number of patients did not allow the detection of any association between the size or type of device and the kind of complication. Cohn and associates14 showed that the complication rate was significantly higher in infants younger than four months as compared to those aged four months to one year. This is consistent with the majority of recently published reports dealing with this matter, which show how complications occur more frequently in children under 10 years old, or five kilograms in weight1-6.
Additional information which has emerged from our study is that complications can occur several months after ASD device implantation. This is confirmed in the literature and, as has also been suggested by others, careful long-term follow-up is mandatory for these patients1,2.
In our study, 18 patients underwent late surgical treatment for various problems linked with the percutaneous procedures. As listed above, thirteen of them were children who required surgery after transcutaneous ASD closure. In six cases the reason was a clinically relevant residual shunt, while in two other patients the reason was erosion of the endocardium by the metal disc of the device with pericardial bleeding and tamponade. A similar mechanism might be postulated for the two patients who developed aortic to right atrial fistula. This is a very rare complication. It seems that a deficient aortic rim and a possible oversizing of the device can contribute to fistula formation15. Moreover, two other patients developed left-sided device thrombosis and required surgery to avoid catastrophic embolic consequences. In another patient, unfortunately it was the device itself which actually broke. Fractures were found in all regions of the wire loop. The main concerns about wire fractures in intracardiac devices are, of course, the perforation of or the damage to adjacent cardiac structures, the formation of thrombus, and embolisation16. All patients underwent surgical treatment with removal of the device, repair of the damaged structure, and ASD closure. Obviously, this kind of surgery has higher operative risks, because bleeding can be massive and the removal of the device challenging.
In the survey by the ECHSA1, it was confirmed that ASD device-related complications leading to surgery do not necessarily occur early after the procedure (i.e., in the cathlab or during the same hospitalisation). In almost one third of cases they occur later in the follow-up. The seriousness of these complications is out of proportion to the severity of the lesion treated and the established track record of its surgical management. Documentation of severe late complications supports the recommendation that, after transcatheter procedures, patients should remain under permanent surveillance to detect potential long-term device-related events. The follow-up in these patients, as stressed in ECHSA guidelines1, should last for at least ten years after the procedure. This last point should be clearly explained to patients and families, while explaining the advantages and drawbacks of transcutaneous ASD closure as compared to surgical treatment13. As a matter of fact, the only conditions that require a long-term follow-up after surgery are: i) repair in adult age, ii) preoperative pulmonary artery hypertension, iii) preoperative or postoperative atrial arrhythmias, iv) preoperative or postoperative ventricular dysfunction, v) coexisting valvular or other cardiac lesions. Patients with ASD who underwent surgical closure in childhood are generally free from late complications17. A remarkable finding of our study was the report of two cases of coronary artery compression (CAC) following TPVI. CAC is a rare, yet well documented, complication related to TPVI and with potentially catastrophic consequences. In larger studies on TPVI, CAC is reported in about 1% to 4.4% of all cases18,19. In a contemporary series of more than 400 patients scheduled for TPVI, about 5% of patients did not undergo valve placement because of a documented risk of CAC (congenital coronary anomaly, tetralogy of Fallot, transposition of the great arteries, patients with re-implanted coronary ostia and abnormal relationship of the RVOT and coronary arteries). Pre-implantation simulation testing with balloon inflation in the RVOT and simultaneous coronary artery angiography is the best way to understand the relationship between the conduit and the coronary arteries. However, there are reports of at least two cases with “negative” balloon simulation tests that failed to predict CAC18,20. In our centre, we recently introduced three-dimensional angiography (3D RDA), which allows a better evaluation of the coronary location, providing additional information to help avoid this catastrophic event. It is also important to emphasise that in the literature20 it has been shown that CAC can occur even months after valve implantation. Thus, meticulous follow-up should be considered after TPVI in selected patients. Moreover, while the mechanism of acute CAC is clearly related to the stent expansion and/or the valve implant, mechanisms of delayed CAC remain elusive. Possible aetiologies include local oedema, which may take a few days to evolve, and/or thrombus formation caused by dilation-related injuries19. In our series, we did not experience any case of delayed CAC. Finally, we report three patients with endocarditis after TPVI during the follow-up period. This complication can also occur after the surgical approach with a similar incidence. For instance, it has been reported that the Contegra® (Medtronic) conduit and the Melody valved stent have a comparable rate of infective endocarditis which has been found to be significantly higher when compared to homograft21.
Limitations
Our study has several limitations. First, there is the retrospective nature of the study. Second, patients were not treated in the same period and the procedures were performed by different physicians. Third, we performed a single-centre study, so the results are limited to our own experience. Furthermore, during a period of longer follow-up, the rate of complications could be different from the one found in our study.
Conclusions
In conclusion, transcatheter interventional procedures are an excellent approach to treat a spectrum of congenital heart diseases with a low, yet not negligible, risk of complications needing surgical treatment. A cardiac transcutaneous device procedure should be performed only in facilities where a cardiac surgical team is available. The chance of developing late complications after device implantation, especially for atrial septal defect device closure, makes a long-term follow-up mandatory for these patients.
| Impact on daily practice Transcatheter approaches are the treatment of choice for several congenital heart defects. However, adverse events may occur during interventional procedures. In these patients the risk of rescue surgery after device release, immediately or months after the procedure, is very low but not negligible and late complications are possible. A cardiac transcutaneous device procedure should be performed only in facilities where an expert cardiac surgical team is available. The chance of developing late complications after device implantation makes long-term follow-up mandatory for these patients. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

