
Abstract
Aims: The feasibility of transcatheter closure of atrial septal defects (ASD) without fluoroscopy has been proven. However, the technique has not been accepted for routine use. We report our experience with transcatheter closure of ASD without fluoroscopy and introduce a well-established technique for alternative use in children.
Methods and results: From November 2013 to May 2015, a total of 114 children with isolated secundum ASD (diameter 6 mm to 24 mm, median 13.8 mm) underwent attempted transcatheter device closure under the guidance of transoesophageal echocardiography without fluoroscopy. Using a modified delivery system and procedure technique, ASD were successfully closed in 110 patients (96.5%, 52 males, 58 females, aged from three to 14 years, median 5.4 years, weight 12 to 46 kg, median 23.5 kg). Procedure time ranged from eight to 42 minutes (median 18 min). Three patients underwent percardiac device closure after failed procedures. In the early stage of our study, before delivery system and technique modification, one patient was converted to open heart surgery due to rupture of the left appendage. No intervention-related complications were detected during a median follow-up of nine months.
Conclusions: Using a modified delivery system and a re-established procedure, we showed that transcatheter closure of ASD without fluoroscopy is a safe technique for alternative use in children.
Abbreviations
ASD: atrial septal defects
ASO: atrial septal occluder
RA: right atrium
TEE: transoesophageal echocardiography
TTE: transthoracic echocardiography
Introduction
Atrial septal defects (ASD) are the third most common type of congenital heart disease, of which about 65~70% are secundum defects1. Improvements in device design and ease of use coupled with avoidance of cardiac surgery have led many centres to adopt transcatheter closure of secundum defects as their first choice1. Current results of device closure of secundum defects show a good safety and efficacy profile2-4. However, radiation exposure in childhood is of more and more concern because children are more highly radiosensitive than adults5,6. To avoid radiation exposure in transcatheter closure of ASD, a fluoroscopy-free technique was developed by Ewert around 20007,8, though the study sample size was relatively small. Unfortunately, since then there has not been any further literature demonstrating the widespread use of this technique. We have established a standard procedure for transcatheter closure of ASD under echocardiographic guidance without fluoroscopy, which has become a routine technique in our centre. Herein we report our experience with this technique in 114 children.
Methods
PATIENTS
From November 2013 to May 2015, a total of 114 children (55 males, 59 females), aged from three to 14 years (median 5.4 years), and weighing from 12 to 46 kg (median 23.5 kg), with isolated secundum atrial septal defects (diameter 6 mm to 24 mm, median 13.8 mm) underwent attempted transcatheter device closure. The ASD were detected by pre-operation transthoracic echocardiography (TTE) (Vivid™ E9; GE Healthcare, Little Chalfont, United Kingdom) with colour and Doppler interrogation from subxyphoid, apical, and parasternal views. Transoesophageal echocardiography (TEE) (Vivid E9; GE Healthcare) was used for an accurate determination of ASD location, morphologic features, size (the largest diameter), and length of rims before the procedure.
The following inclusion criteria were applied: 1) secundum ASD with symptomatic or evidence of right heart volume overload; 2) sufficient rims (≥5 mm from the ASD to the superior or inferior vena cava, right upper or lower pulmonary vein, coronary sinus, mitral or tricuspid valve); 3) the diameter of the left side of occluder not longer than the overall length of the atrial septum; 4) no other associated cardiac anomalies requiring surgical repair; 5) age ≥3 years; 6) weight ≥12 kg.
All procedures were in accordance with institutional guidelines and were approved by the institutional review committee. Study approval was obtained from the Committee on Clinical Applications at the Second Xiangya Hospital and informed consent was obtained from the patients’ parents.
DEVICE
The SHSMA atrial septal occluder (ASO) is a self-centring device made from a nickel titanium alloy (Shanghai Shape Memory Alloy Co. Ltd., Shanghai, China), which is authorised for use by the Food and Drug Administration of China. The delivery system has been modified for safe use in this new procedure. The delivery sheath has been shortened, the dilator is 2 cm longer than the sheath, and there is a scale on the sheath (Figure 1).
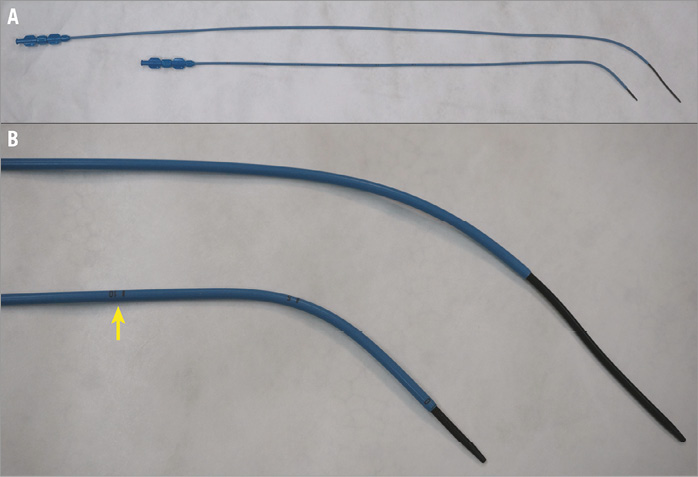
Figure 1. Comparison of two delivery systems. A) Traditional delivery system above and modified delivery system underneath. B) Magnified image showing scale (arrow) on modified sheath.
PROCEDURE
The procedure was performed in a routine operating room under TEE guidance without fluoroscopy. All patients underwent general anaesthesia with intubation. TEE was applied to perform a comprehensive study after intubation, looking at all aspects of the ASD anatomy (location, size, presence of additional defects, and adequacy of the various rims). As described previously9, the defect was sized according to the maximum defect diameter. The occluder for each patient was selected in accordance with the TEE result, with a diameter 2 to 4 mm in excess of the maximum defect diameter. Then the distance between the infrasternal angle and the middle of the right groin of the patient (the so-called “safe distance”) was measured by the operator. Vascular access was obtained through the right femoral vein. Heparin at a dose of 100 IU/kg and antibiotic prophylaxis were administered. A 0.035-inch J-tipped stiff guidewire (Terumo Medical Corporation, Somerset, NJ, USA) was advanced into the right atrium (RA) under TEE guidance using a bicaval view. A 5 Fr MPA 2 diagnostic catheter (Cordis, Johnson & Johnson, Warren, NJ, USA) was subsequently advanced into the RA along the wire. Then the guidewire was withdrawn progressively and the tip of the catheter was tracked by TEE. Clockwise torque on the catheter may be needed to obtain the right direction whilst advancing through the defect towards the left atrium and the left upper pulmonary vein. Guided by the catheter, the guidewire was then advanced through the defect and into the left upper pulmonary vein, and the catheter was extracted (Figure 2A, Figure 2B). The delivery sheath and dilator were advanced into the femoral vein over the guidewire, with TEE monitoring the inferior vena cava using the bicaval view. As soon as the sheath and dilator were detected by TEE, the dilator was pulled back into the sheath to get a clear image of the tip of the sheath (Figure 2C). If the sheath and dilator have entered further than the “safe distance” but TEE does not detect them, adjustment of the TEE view or retraction of the sheath should be considered. Tracked by TEE, the sheath was then advanced to the left upper pulmonary vein (Figure 2D). A loading sheath with an appropriately sized ASO was introduced into the delivery sheath until it reached the tip of the sheath. Under TEE guidance, the sheath was then gently withdrawn to deploy the left disc and waist in the left atrium and then pulled back until the disc was against the atrial septum (Figure 3A). The right side of the device was deployed by retracting the delivery sheath while applying slight tension on the cable (Figure 3B). Before the device was unscrewed, its correct and stable position was confirmed by TEE, demonstrating unobstructed flow from the coronary sinus, pulmonary veins and superior and inferior cava veins and competence of the atrioventricular valves. Aspirin (3 mg/kg/day) was routinely administered for six months. The patients were followed up by clinical examination, electrocardiography and TTE at two weeks, one month, three months and six months, and then yearly.
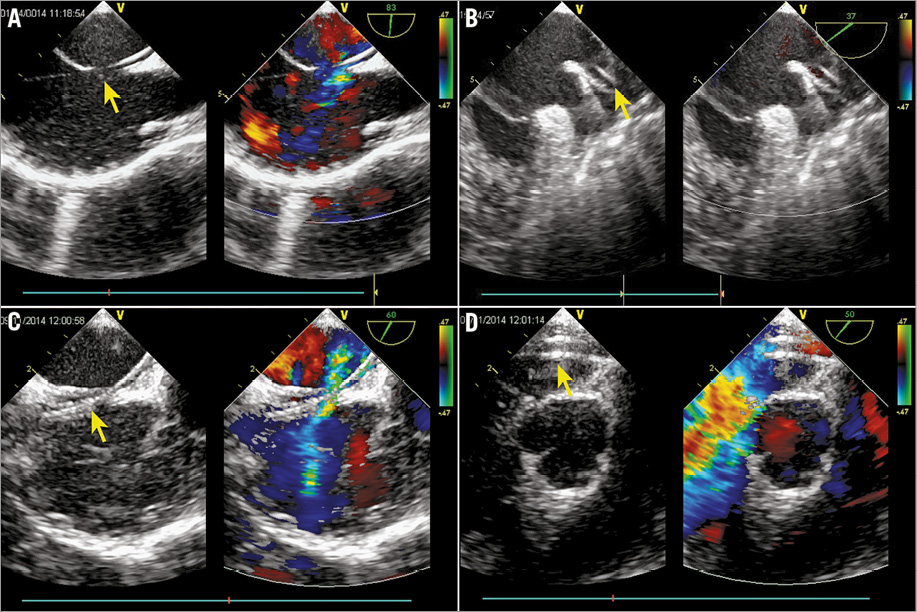
Figure 2. Delivery tract establishment. A) & B) Guidewire (arrow) advanced through the defect and into the left upper pulmonary vein under TEE guidance. C) Tip of the sheath (arrow) clearly detected by TEE. D) Sheath (arrow) advanced to the left upper pulmonary vein tracked by TEE.
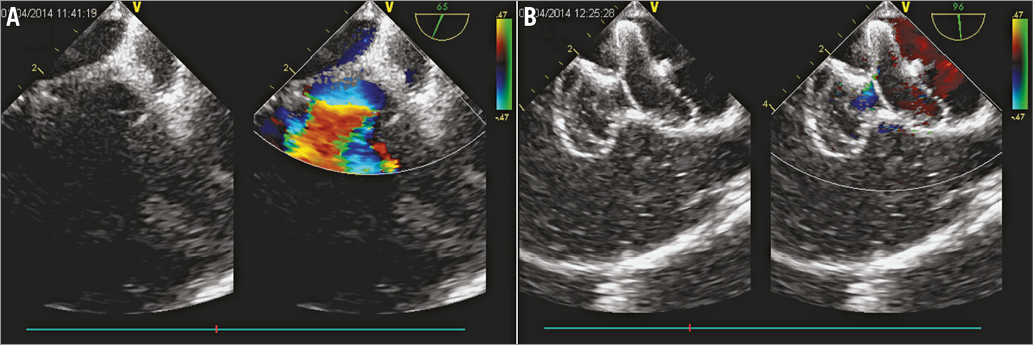
Figure 3. Device deployment. A) Left disc of the ASO was deployed in the left atrium. B) Right side of the device was deployed by retracting the delivery sheath while applying slight tension on the cable.
Results
From November 2013 to May 2015, a total of 114 paediatric patients with isolated secundum ASD underwent attempted transcatheter device closure under TEE guidance only. All procedures were carried out in a standard theatre without fluoroscopy back-up, but with a cardiopulmonary bypass unit stand-by. The transcatheter occlusion procedure under TEE guidance was successful in 110 (96.5%) patients. Three patients were converted to percardiac device closure as described previously10, after failed attempts at transcatheter closure due to a short and soft rim to the inferior vena cava. In the early stage of this study, before delivery system and technique modification, rupture of the left appendage occurred in one child who underwent surgical repair due to acute cardiac tamponade. There were no other acute procedural complications or severe adverse events (e.g., death, valve injury, device drop-off, or embolism). The procedure time ranged from eight to 42 minutes (median 18 minutes). Correct placement of the device at the first attempt was achieved in 92 of 110 patients. In the remaining 18 patients, redeployment of the ASO was necessary because the device was perpendicular to the atrial septum, so that both discs were deployed in the left or right atrium. During the second deployment, the following adjustments could be helpful: 1) obtaining an appropriate angle between the sheath and atrial septum by clockwise torque on the sheath; 2) deploying the left disc, waist and part of the right disc in the left atrium before pulling back to the atrial septum. Immediate complete closure of ASD was achieved in 96 patients (87%), trivial shunts (diameter <1 mm) in 12 (11%), and small shunts (1-2 mm) in two (2%) patients. A transient, well-tolerated episode of junctional rhythm was observed in four patients (4%) during the operation. There were no other immediate procedural complications or severe adverse events (e.g., death, valve injury, device drop-off, or embolism). At discharge, neither residual shunting nor dislocation of the device was detected. Incomplete right bundle branch block was found in eight patients (7%).
All the patients were followed by clinical examination, echocardiography, and transthoracic echocardiography. No severe adverse events (death, valve injury, complete atrioventricular block, embolism, or ventricular outlet stenosis) were noted during the period from two to 19 months (median nine months).
Discussion
Since King and colleagues11 reported the first transcatheter device closure of a secundum defect in 1976, the development of fluoroscopically guided catheter-based interventions has revolutionised the management of congenital heart disease12. The transcatheter occlusion procedure has been considered the primary mode of treatment for patients with secundum ASD in many centres1, because it has been regarded as generally safe and effective4. The traditional intervention is performed under fluoroscopic and angiographic guidance, which can offer excellent visualisation and tracking performance. However, the radiation exposure issue in cardiac catheterisations, especially therapeutic cardiac catheterisation, is particularly relevant for infants and children because of their higher radiosensitivity compared with adults, the large fraction of the body irradiated by the X-ray beam, and the probable need to repeat the procedure13,14. Moreover, radiation exposure in childhood may significantly increase lifetime cancer risk concern because children have immature developing organ and tissue structures, and may potentially have a longer lifespan5,6,15.
In an effort to reduce radiation exposure, Ewert and colleagues successfully performed transcatheter closure of ASD under the guidance of echocardiography alone7,8. However, this technique has not been widely accepted for routine use for unknown reasons. We suppose that the safety of this technique may be a big issue. The difference of the operator’s performance view may account for this. Unlike the traditional procedure, the performance view in the fluoroscopy-free technique is TEE, which only provides two-dimensional (2D) views for operators. Although TEE has shown more advantages over fluoroscopy in the measurement of rims and dimensions of ASD, monitoring the deployment of the ASO and evaluation of the result of the implant16-19, the biggest challenge for operators is to track the guidewire and sheath under the 2D view. Our initial experience confirms this. In the early stage of our study, acute cardiac tamponade occurred in one child due to the rupture of the left appendage injured by the tip of the dilator, something which cannot be detected by echo. Also, a lot of time was needed to track the catheter and sheath during the procedure. Therefore, we modified the delivery system as shown in Figure 1 and established a standard procedure. After that, the procedure time was obviously shortened and no intervention-related complication occurred in subsequent patients. Another security strategy was also undertaken in our study. We recommend the “safe distance” which refers to the distance from the right femoral vein to the right atrium. If the safe distance is approached while the sheath cannot be detected, any blind performance should be prohibited. Based on these modifications and security strategies, an excellent result has been achieved in our study.
Visualisation of deployment is also important for the safety and efficacy of device closure of ASD4. In our study, the device placement process was clearly visible using TEE for guidance, and well-timed placements can be precisely controlled by the operators. Any dislocation or residual shunting is easy to find and further adjustments can be made immediately. Complications, such as interfering with flow from the right superior pulmonary vein, or with the normal function of the atrioventricular valves, can also be detected simultaneously. The procedure time for the fluoroscopy-free transcatheter ASD closure ranged from eight to 42 minutes in our study. The main factors associated with procedure time include: 1) the process to reach the pulmonary veins, because there may be some difficulty to track the tip of the catheter and/or guidewire with TEE; 2) the course of device deployment, as the angle between the sheath and atrial septum may not be good enough, so that redeployment may be needed. Although a longer operating time was needed in certain cases, especially in the early stage of the study, the procedure time decreased remarkably as operators gained experience. As shown in Figure 4, the learning curve is very short for operators, especially for surgeons.
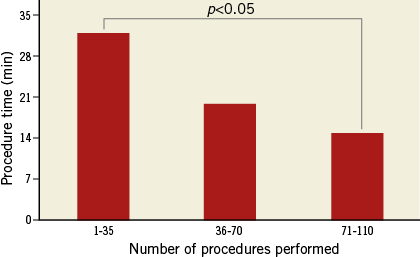
Figure 4. Learning curve. Data analysed with ANOVA test.
Device embolism is the most frequent complication of percutaneous transcatheter closure of ASD, which could be lethal4. Proper selection of patient and device is mandatory for prevention of this inherent complication. In our study, all procedures were carried out in a standard theatre with a cardiopulmonary bypass unit stand-by. Thus, a patient could be converted to open heart surgery immediately in order to retrieve the device and repair the ASD. A portable C-shaped arm X-ray system is also backed up for accurate location of the device in case of embolisation. After ASD device closure, patients were also followed very carefully by echocardiography. No embolism event occurred in the current study series.
In the current study, patient selection criteria were similar to the traditional approach and a similar success rate was achieved2. One possible disadvantage of this technique is that the procedure is performed under general anaesthesia. However, we consider this only a minor drawback because general anaesthesia might also be needed for young children when performing traditional transcatheter closure of ASD. Our experience was still limited, and longer follow-up is needed in future research. This study was limited to one institution, and other institutions may find different results.
Conclusions
In conclusion, our study demonstrated that the modified fluoroscopy-free technique for transcatheter closure of ASD is a safe and feasible technique which can be applied for routine use, especially for children.
| Impact on daily practice Radiation exposure in childhood is of more and more concern because children are more highly radiosensitive than adults. Using a modified delivery system and a re-established procedure, transcatheter closure of ASD under TEE guidance is a safe technique for alternative use in children. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

