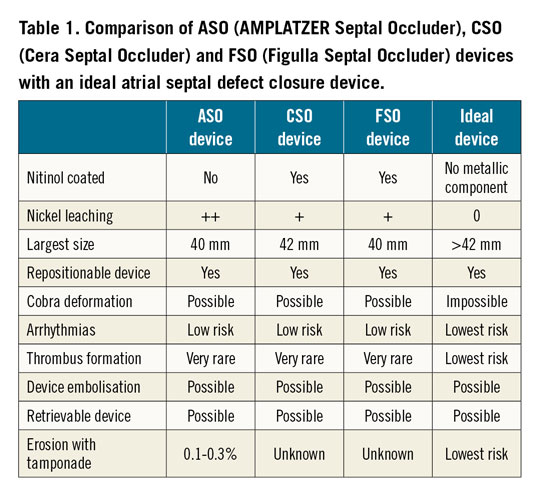
Usually, the commercial life of an implantable medical device is limited, rarely exceeding ten years in the absence of technological upgrading. The AMPLATZER™ Septal Occluder (ASO; Abbott/St. Jude, St. Paul, MN, USA) is an exception to the rule. The ASO is a transcatheter closure device intended for the percutaneous occlusion of atrial septal defects (ASD) which has been available on the market since 19981. To our knowledge, there have been no relevant changes to the ASO device, a braided nitinol self-centring double disc device designed by Dr Kurt Amplatz more than 20 years ago. Was the ideal device created? Obviously, for almost a decade, other ASD occluders have been developed with a design quite similar to the ASO device, such as the Cera™ Septal Occluder (CSO; Lifetech Scientific [Shenzhen] Co., Ltd, Shenzhen, China) and the Figulla® Septal Occluder (FSO; Occlutech, Helsingborg, Sweden)2,3. Several technical characteristics differentiate these occluders from the ASO in terms of device design, coating and disc size with theoretical advantages in terms of clot formation, nickel leaching, cobra deformation, atrial arrhythmias and cardiac erosion. However, and probably not surprisingly due to the rarity of complications, these three devices offer similar efficacy, safety and outcomes in the hands of experienced operators, as demonstrated by Bhattacharjya et al in this edition of EuroIntervention4.
In this study, the three occluders were serially allocated in a cycle of three to 450 consecutive patients undergoing transcatheter ASD closure in a single institution. Patients were comparable at baseline in all parameters and follow-up duration was 12-47 months. Early results showed no major complications and procedural success was 99.6%. The defect and device sizes were similar in all groups. The delivery system was significantly smaller with the ASO. The FSO needed special deployment techniques less often and formed a cobra deformity more often, though this was not statistically significant. Patient outcome was similar among the three groups. None of the patients presented with new significant pericardial effusion, new-onset arrhythmia, stroke, cardiac perforation, device erosion, or embolisation during the one- to four-year follow-up. The authors should be congratulated for having successfully performed this important study confirming the very high success rate of transcatheter ASD closure in experienced hands with a very low rate of complications. However, despite the inclusion of 450 consecutive patients, this study was not powered to evaluate the rare (0.1-0.3%) but dramatic risk of erosion and tamponade5.
In fact, the currently available occluders are probably close to the ideal device (Table 1). After a learning curve using the same device, a fairly experienced operator could use the other devices. However, in some countries, the volume of percutaneous ASD closures by operator is relatively low and this does not make it easy to use different occluders.
Cardiology has a culture of randomised clinical trials and evidence-based medicine. Another characteristic of the transcatheter ASD closure story is the total absence of randomised controlled data. Naturally, percutaneous treatment has been adopted as it offers an excellent alternative to surgery for ASD with a favourable anatomy. All implantable medical devices must be evaluated continuously, even the oldest, and obviously also the most recent. Efforts to increase the evidence should continue by establishing large well-designed multicentre registries. Potential device-based differences could then be shown in the future regarding the risk of tamponade6.
Conflict of interest statement
P. Aubry has received consultant fees/honoraria from Occlutech. E. Brochet has received proctor/consultant fees from Abbott Vascular. J. Juliard has no conflicts of interest to declare.

