Abstract
The GORE CARDIOFORM atrial septal defect (ASD) Occluder (GCA) is composed of a platinum-filled nitinol wire frame covered with expanded polytetrafluoroethylene, making it softer and more conformable compared with nitinol mesh devices. After the ASSURED clinical study confirmed the efficacy and safety of the device, it received U.S. Food and Drug Administration approval and a European conformity mark. Our aim was to understand the learning curve implicated in using the GCA for ASD closure in paediatric and adult patients as well as to study the early outcomes. To this end, a review of ASD device closures with GCA in 4 UK centres was conducted between January 2020 and January 2023. Implantation success was the primary outcome; the secondary outcomes were serious adverse events, including new onset arrhythmia. In all, 135 patients were included, and 128 (95%) had successful ASD device closure with GCA. The median patient age was 49 years, the median defect size was 18 mm, and the median device size was 37 mm. The median follow-up time was 6 months (interquartile range 1-14). One device embolisation occurred, and 15 patients (12% of GCA implantations) developed new onset arrhythmia – this was not related to patient age, defect diameter or device oversizing but was positively associated with device size. With growing experience using GCA, the device can be applied to a wide variety of ASD sizes and morphologies. Given the number of successful implantations with an absence of aortic erosion, as well as the ability to perforate through the device should procedures be required in the left atrium, the GCA device is an important addition for interventionists who close atrial septal defects.
Introduction
The GORE CARDIOFORM atrial septal defect Occluder (GCA; W.L. Gore & Associates) is composed of a platinum-filled nitinol wire frame covered with expanded polytetrafluoroethylene, making it softer and more conformable compared with nitinol mesh devices12. It has an anatomically adaptable waist, which fills defects between 8 and 35 mm, expanding to conform to the atrial septal defect (ASD) size and shape12. Therefore, and uniquely, each device size can be used to treat a range of ASD diameters2.
After the ASSURED clinical study confirmed the efficacy and safety of the device, it received U.S. Food and Drug Administration (FDA) approval and a CE (European conformity) mark. Following this, the device was made available for ASD closure in selected centres in the UK in 2020. As the deployment technique and device design differ significantly from previous ASD occluders, we anticipated a learning curve which would impact procedural success and outcomes. A total of 125 cases were included in the ASSURED study; our aim was to assess a comparable size cohort of patients referred for ASD closure in the UK and report the outcomes with the new device. Primarily, we sought to examine whether the ASSURED trial results were reproducible in everyday practice.
Methods
Study population and design
We performed a retrospective review of all cases where GCA was used for attempted ASD device closure in 4 UK centres between January 2020 and January 2023. These were, at the time, the only GCA implantation centres in the UK. Baseline demographic data, procedural details and adverse events during the procedure and follow-up were collected.
The primary outcome was implantation success. Serious adverse events (defined as new clinical symptoms requiring medical attention) during follow-up were documented as secondary outcomes. New onset arrhythmia was defined as a documented arrhythmia in a patient with no previous history, thereby excluding recurrence of previous arrhythmias.
Procedural details
All procedures were performed under general anaesthesia with fluoroscopy and transoesophageal echocardiography (TOE). The defect was balloon sized, and the diameter at the stop-flow was used for device sizing according to manufacturer guidelines, taking into consideration rim deficiency. The first device of choice was the GCA. If the initially selected device failed to close the ASD, either another size GCA was chosen, or a different type of device was used, at the discretion of the operator.
Specific follow-up protocols were set up by individual centres but, in all cases, consisted of electrocardiogram (ECG) and transthoracic echocardiography monitoring at 24 hours and at 4-6 weeks after the procedure and then again at 6-12 months, followed by a review every 1 or 2 years. Routine fluoroscopy to monitor for wire frame fractures was not undertaken unless there was concern about device stability. Antiplatelet therapy (single or dual, depending on operator preference) was prescribed for 6 months after the procedure to prevent thrombus formation during the device endothelialisation phase.
Statistical analysis
GraphPad Prism (version 9.5.1; GraphPad Software) was used for statistical analysis. Medians with interquartile range (IQR) are reported for continuous variables. Unpaired t-tests were used to compare the group of new onset arrhythmias and the group without new arrhythmias after GCA implantation. A p-value less than 0.05 was considered statistically significant. Multiple logistic regressions were applied for analysis of the impact of chosen variables on the incidence of new onset arrhythmias and are reported as odds ratio (OR) with 95% confidence intervals (CI).
Results
Baseline demographics and clinical characteristics
There were 135 procedures with an intention to close an ASD with a GCA reported. The median age at intervention was 49 years (IQR 33-60). Twenty patients (15%) were children and adolescents (less than 18 years of age), and the youngest patient was 4 years old (Central illustration, Table 1, Figure 1).
The median largest defect diameter on balloon sizing at the stop-flow was 18 mm (IQR 15-21), and the maximum defect size that was successfully closed was 27 mm. A total of 64 defects (47%) had deficient rims: the aortic rim was deficient in 56 cases (41%), and the posterior/inferior rim in 12 (9%). Multiple defects were seen in 14 cases (10%).
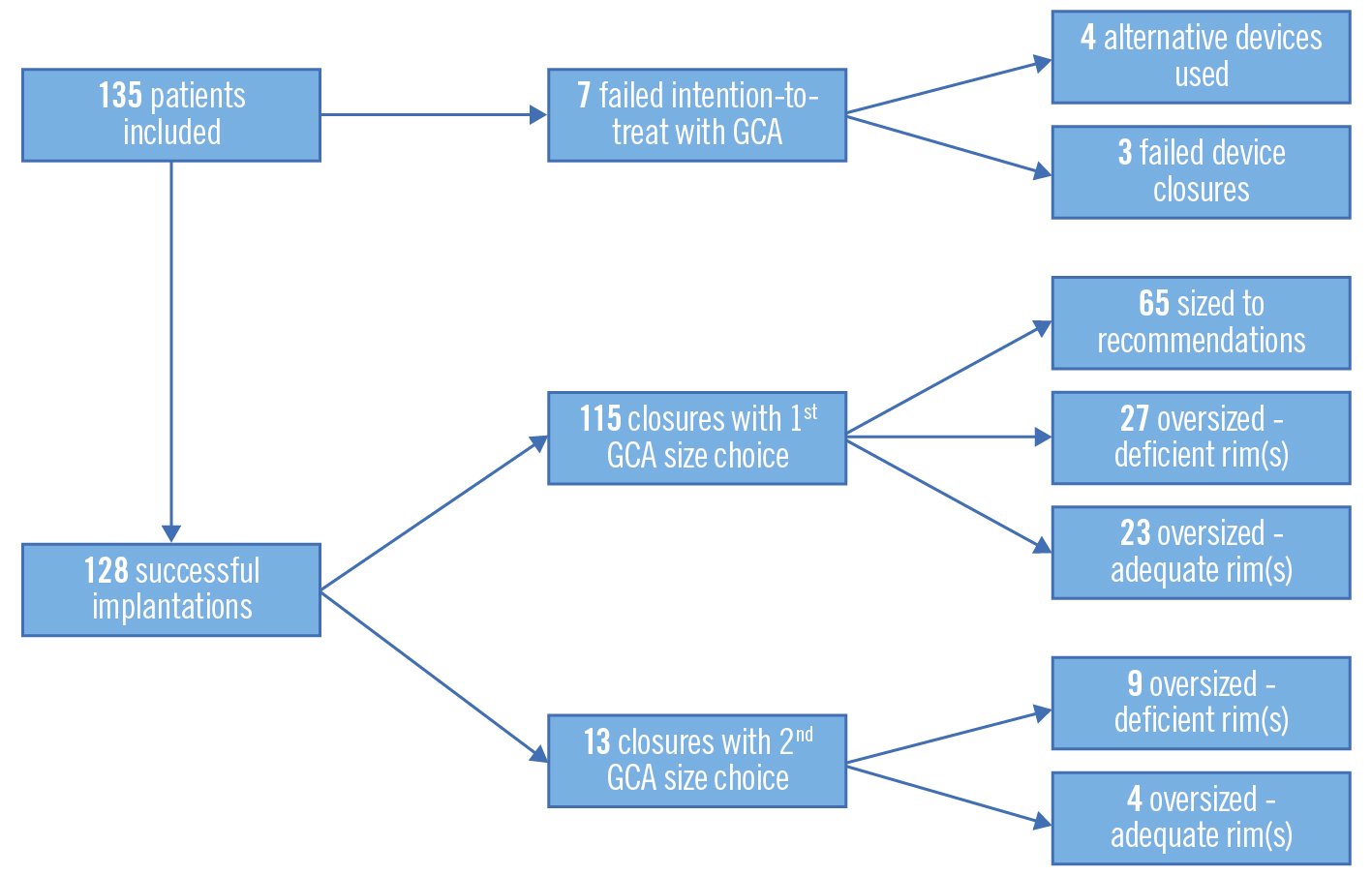
Central illustration. Intention-to-treat flowchart. Successful device implantation was defined as an implanted GORE CARDIOFORM ASD Occluder (GCA) that was not retrieved. Failed intention-to-treat was defined as either an alternative type of device implanted or failed device closure. The device was sized according to manufacturer recommendations (using the diameter of the waist on balloon sizing), oversized (device size larger than manufacturer recommendations) due to deficiency of defect rim(s), or oversized despite adequate defect rim(s).
Table 1. Clinical and procedural data summary.
| Age, years | 49 (33, 60) | ||
| Age <18 years | 20 (15) | ||
| Largest defect diameter, mm | 18 (15, 21) | ||
| Deficient rims | 64/135 (47) | ||
| Aortic rim deficiency | 56/135 (41) | ||
| Posterior/inferior rim deficiency | 12/135 (9) | ||
| Multiple defects | 14/135 (10) | ||
| Intention-to-treat success | Closure with first GCA choice | 115/135 (85) | |
| Closure with second GCA choice | 13/135 (10) | ||
| Closure with alternative device | 4/135 (3) | ||
| Failed procedure | 3/135 (2) | ||
| Implanted device | GORE CARDIOFORM ASD Occluder | 128/135 (95) | |
| Figulla Flex II ASD Occluder | 4/135 (3) | ||
| AMPLATZER Septal Occluder for additional defect | 1/135 | ||
| Implanted GCA size, mm | 37 (32, 44) | ||
| 27 mm | 9/128 (7) | ||
| 32 mm | 23/128 (18) | ||
| 37 mm | 55/128 (43) | ||
| 44 mm | 28/128 (22) | ||
| 48 mm | 13/128 (10) | ||
| Device sizing | Device sized to manufacturer recommendations | 65/128 (51) | |
| Device oversized (deficient defect rims) | 36/128 (28) | ||
| Device oversized (adequate defect rims) | 27/128 (21) | ||
| Procedural complications | Arrhythmia requiring DC cardioversion | 4/135 (3) | |
| Mild pulmonary haemorrhage due to wire injury | 1/135 | ||
| Device embolisation | 1/135 | ||
| Data are expressed as median (range) or n (%). DC: direct current; GCA: GORE CARDIOFORM ASD Occluder | |||
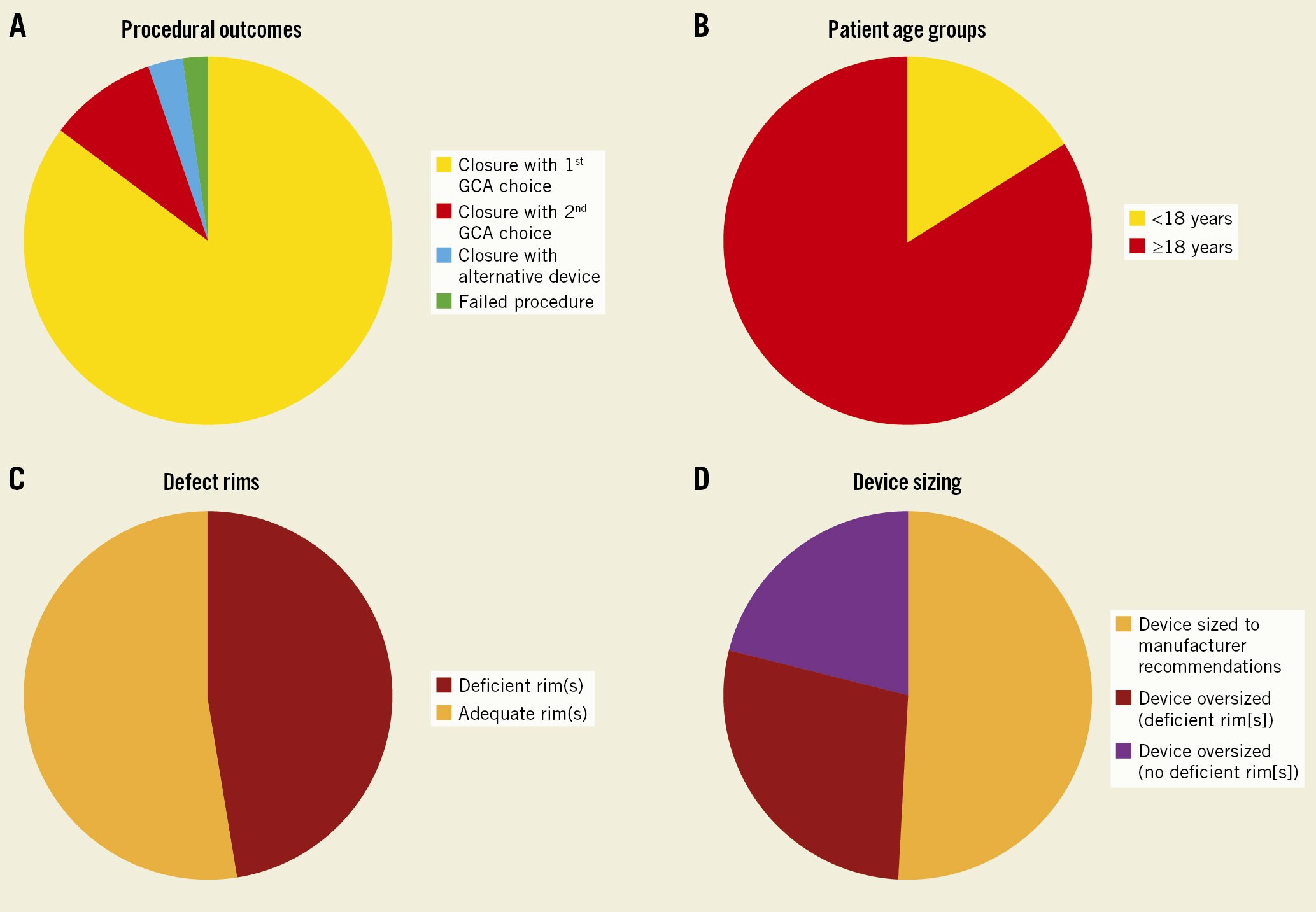
Figure 1. Graphic representations. Graphic representation of procedural outcomes (A), patient demographics (B), defect characteristics (C) and device sizing (D).
Procedural outcomes
In all, 128 cases (95%) were successfully occluded with the GCA. The median GCA device size was 37 mm (IQR 32-44). The device was “oversized” (the chosen size was larger than manufacturer recommendations according to defect diameter on balloon sizing) in 63 patients (49%): 36 of these had a defect with deficient rims, while no rim deficiency was present in 27 patients with oversized devices. In 18 procedures (13%), a second device, replacing the first selected device, was used to occlude the defect due to the initial device prolapsing, an inability to secure the rims, or a residual shunt. The GCA was upsized in 13 patients, and in 4 cases, an alternative nitinol mesh device (Figulla Flex II ASD Occluder; Occlutech) was selected (3%). An AMPLATZER Septal Occluder (Abbott) was used in addition to a GCA for a residual defect in 1 patient. One patient was referred for surgical closure due to a large residual shunt even after changing from a GCA to an alternative device. Two patients had a failed procedure with a GCA and no other device size or type was used in its place (Table 1, Figure 1).
Six patients had a residual shunt, three of those due to additional defects. All the residual shunts were considered haemodynamically insignificant, as assessed by TOE and the postprocedural follow-up echocardiograms.
Periprocedural complications occurred in 8 patients. One device embolisation occurred within 24 hours of the procedure, requiring surgical device retrieval and defect closure. The device was oversized in this patient despite adequate rims (15 mm defect, 37 mm device). No other procedural complications occurred, except for 1 case of mild pulmonary haemorrhage related to a pulmonary vein wire injury that was successfully managed conservatively. Four patients developed atrial fibrillation/flutter during the intervention, requiring direct current (DC) cardioversion. There was a case of a transient type II heart block that resolved after general anaesthesia was reversed. One patient reported lower limb weakness and numbness likely due to sciatic nerve compression or to an inadvertent regional nerve block (brain computed tomography excluded a cardiovascular accident) that resolved spontaneously.
Follow-up
The median follow-up time was 6 months (IQR 1-14). Adverse events occurred in 18 patients (14%) after GCA implantation, 9 (7%) of these within 30 days post-procedure. Apart from 3 cases of persistent new onset arrhythmias (2% of GCA implantations), all other adverse events had no long-term consequences. The median rate of peri- and postprocedural cardiac adverse events was 0 events per person.
New onset arrhythmias
New onset postprocedural arrhythmia (atrial flutter/fibrillation/supraventricular tachycardia) was documented in 15 patients (12% of ASD closures with GCA): 12 resolved either spontaneously or with treatment and 3 continue to have paroxysms despite medical treatment (Figure 2). Patient details are shown in Table 2. The patients with new onset arrhythmia were older (none occurred in patients under 18 years of age), had larger defects and received larger devices (Table 3); none of these differences reached statistical significance. Multiple logistic regression analysis showed no correlation between new arrhythmias and age at the procedure, defect diameter, or device oversizing (Table 4). There was a weakly positive relationship between device size and development of new arrhythmia post-implantation (OR 1.11, 95% CI: 1.01-1.23).
Ten patients (8%) reported palpitations post-procedure that spontaneously resolved, and no further investigations, such as 24-hour ECG monitoring, were undertaken in these cases. Six patients developed recurrence of paroxysmal atrial fibrillation despite ablation preprocedure: 2 were resolved and 4 are ongoing.
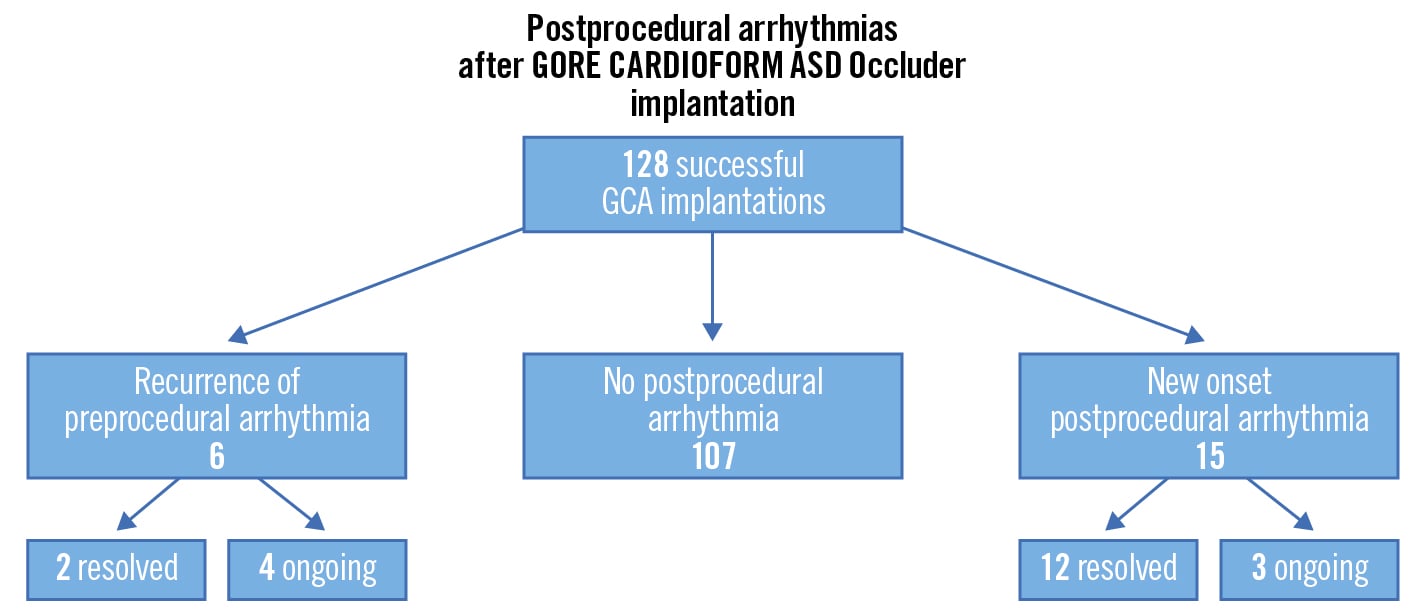
Figure 2. Incidence of arrhythmias after successful GCA implantation. Fifteen patients (12%) developed a new atrial/supraventricular arrhythmia. ASD: atrial septal defect; GCA: GORE CARDIOFORM device
Table 2. New onset postprocedural arrhythmia and other serious adverse events after GORE CARDIOFORM ASD Occluder implantation.
| Age (years) | Defect size (mm) | Implanted device size (mm) | Serious adverse event | Outcome |
|---|---|---|---|---|
| 51 | 21 | 44 | Paroxysmal atrial fibrillation | Ongoing (on verapamil) |
| 46 | 17 | 37 | Supraventricular tachycardia | Resolved (spontaneous cardioversion) |
| 30 | 20 | 44 | Atrial flutter, atrial tachycardia | Resolved (on bisoprolol) |
| 62 | 22 | 48 | Paroxysmal atrial flutter | Ongoing (on bisoprolol) |
| 49 | 18 | 37 | Paroxysmal atrial fibrillation | Ongoing (on flecainide) |
| 53 | 13 | 32 | Supraventricular tachycardia (re-entry) |
Resolved (ablation) |
| 51 | 18 | 37 | Haemoptysis and fever 2 days post-procedure (?pneumonia) | Resolved |
| 64 | 23 | 37 | Migraines | Resolved |
| 55 | 15 | 37 | Atrial tachycardia | Resolved (on bisoprolol) |
| 54 | 25 | 44 | Atrial flutter | Resolved (on bisoprolol) |
| 61 | 24 | 48 | Atrial tachycardia | Resolved (on bisoprolol) |
| 55 | 20 | 44 | Atrial fibrillation | Resolved (on bisoprolol) |
| 54 | 23 | 44 | Atrial fibrillation | Resolved (cardioversion with bisoprolol) |
| 38 | 15 | 32 | Atrial fibrillation | Resolved (DC cardioversion) |
| 73 | 18 | 37 | Atrial fibrillation | Resolved (DC cardioversion) |
| 55 | 18 | 44 | Atrial fibrillation | Resolved (spontaneous cardioversion) |
| 55 | 21 | 44 | Atrial flutter | Resolved (spontaneous cardioversion) |
| 50 | 24 | 48 | Heart failure − admission required, settled with diuretics | Resolved |
| 41 | 15 | 37 | Device embolised at 24 hours | Surgical retrieval and ASD repair |
| ASD: atrial septal defect; DC: direct current | ||||
Table 3. Clinical and procedural characteristics comparison between patients who developed a new onset postprocedural arrhythmia and the remainder of the patients after GCA implantation.
| New postprocedural arrhythmia (n=15) | No new postprocedural arrhythmia (n=113) | p-value | |
|---|---|---|---|
| Age, years | 50 (47, 53) | 47 (30, 60) | 0.37 |
| Defect size, mm | 19 (17, 21) | 18 (15, 20) | 0.45 |
| Device size, mm | 41 (37, 44) | 37 (32, 44) | 0.23 |
| Data are expressed as median (range). ASD: atrial septal defect; GCA: GORE CARDIOFORM ASD Occluder | |||
Table 4. Multiple logistic regression analysis of potential influencers on new arrhythmia development after GCA implantation.
| Variable | Odds ratio | 95% confidence interval |
|---|---|---|
| Age | 1.02 | 0.99-1.06 |
| Defect size | 1.10 | 0.97-1.25 |
| Device size | 1.11 | 1.01-1.23 |
| Oversizing (with or without deficient rims) | 1.58 | 0.53-4.99 |
| Oversizing without deficient rims | 1.96 | 0.56-6.10 |
| ASD: atrial septal defect; GCA: GORE CARDIOFORM ASD Occluder | ||
Other postprocedural adverse events
One patient presented with haemoptysis and fever 2 days after the procedure and was treated for pneumonia. One case reported new migraine onset. There was a case of a clinical picture of heart failure after ASD closure that responded to diuretics in a 50-year-old patient.
Discussion
Implantation success
The ASSURED study reported 96% technical success2. Our results align with these findings; 95% of patients in whom GCA implantation was attempted had successful device closure with the device. There was a difference in patient demographics in our study, with predominantly adult patients in our cohort (85% of patients were older than or equal to 18 years old), compared to the predominantly paediatric population in the US study (72% were less than 18 years old). The median defect size is similar (18 mm in our study vs 17 mm in the ASSURED study). The implanted devices in our cohort, however, showed a shift towards selecting larger sizes: the most common device size was 37 mm, followed by 44 mm, while in the ASSURED study, the 32 mm size was used most frequently, followed by the 37 mm.
Device oversizing was common and occurred in half of the successful implantations. Rim deficiency was present only in 57% of these patients, and there were 4 cases where the device had to be oversized due to initial failure to occlude the ASD with the chosen size despite adequate rims. Less experienced operators may have a tendency to oversize in an effort to reduce the risk of embolisation; however, this should be balanced with a possible increased risk of wire frame fractures and arrhythmias, as discussed later.
Serious adverse events
The rate of serious adverse events in the ASSURED study was low (4.8% at 30-day follow-up) and remains relatively low in our cohort (7% at 30 days). Only 1 device embolisation occurred; this was a somewhat unexpected finding. Traditional stability checks that can be performed with nitinol mesh devices (“push and pull” manoeuvres before device release) are possible with GCA but, given the device is soft, are not as informative. Careful interrogation of the device both with fluoroscopy and TOE before locking and before GCA release is more crucial for assessment of rim capture and device stability. The rate of device embolisation in this study is low at 0.8% and is consistent with studies of other introduced devices that report the frequency of ASD device embolisation at 0.55% to 1.44%34.
Arrhythmias
In previous reports, new onset arrhythmias have been associated with increasing patient age, larger device implantation and device oversizing2. It has been speculated this is a reflection of patient characteristics rather than the device itself2. Our study adds insights into what might be expected in a predominantly adult population (the median patient age in the ASSURED study was 12 years, compared to 49 years in our cohort). The occurrence of new arrhythmias in our study compared to the ASSURED study was significantly higher (12.0% vs 6.4%). Studies on the occurrence of atrial tachyarrhythmias after percutaneous ASD closure with other devices report an overall incidence of new arrhythmias at 8.6% (95% CI: 4.8-14.9)5. Within our cohort, we found no association between new postprocedural arrhythmias and age, defect diameter or device oversizing; there was a weak positive correlation with implanted device size.
Wire frame fracture and erosion
One of the most important differentiators of the GCA’s design, when compared to nitinol mesh devices, is its conformability, which is intended to minimise wall injury. Device erosion has been a significant concern with all previously approved ASD occluders26; the exception is an international follow-up study on the Cocoon ventricular septal occluder (Vascular Innovations), which reports no erosions to date4. The authors speculate that this could be explained by the soft device design (nitinol mesh covered with a nanoplatinum coating). No erosions have been reported after GCA implantation thus far1, and although still relatively new, there have been a large number of implantations in the USA since the ASSURED study. Wire frame fracture was reported in 36% of patients during the follow-up of the ASSURED study. As this did not result in any clinical sequelae, routine fluoroscopy is not part of the follow-up recommendations in the UK and can be considered a limitation of the study. Although fractures were thought to be clinically insignificant in the earlier-generation Gore patent foramen ovale (PFO) device (GORE HELEX Septal Occluder; W.L. Gore & Associates)7, 2 cases of late wire perforations due to fracture resulted in cardiac tamponade after GORE CARDIOFORM Septal Occluder (GSO) implantation8 (wire frame fracture has been reported in 6.8% of GSO implants9). Since device oversizing is also speculated to be one of the mechanisms that can lead to deformation and frame damage, cautious device size selection to accommodate anatomical challenges and minimise both wire fracture and arrhythmia risks is advocated.
Limitations
None of the UK centres performed screening fluoroscopy to monitor for device wire frame fracture. Fractures remain a concern, even though no clinical consequences have yet been seen. Long-term follow-up of GCA implants will give more insight into this. Routine 24-hour ECG monitoring after device implantation was not performed, and therefore, arrhythmias in patients with palpitations post-procedure that spontaneously resolved might not have been documented.
Conclusions
Our experience confirms that the GCA device can be used to occlude a wide variety of ASD sizes and morphologies (Figure 3). All included centres have extensive experience with ASD device closure − GCA has performed at least comparably, if not in some aspects superior, to other available devices. Given the number of successful implants in the USA with an absence of aortic erosion as well as the ability to perforate through the device should procedures be required in the left atrium10, the GCA device is a useful adjunct to an interventionist’s armamentarium for closing atrial septal defects.
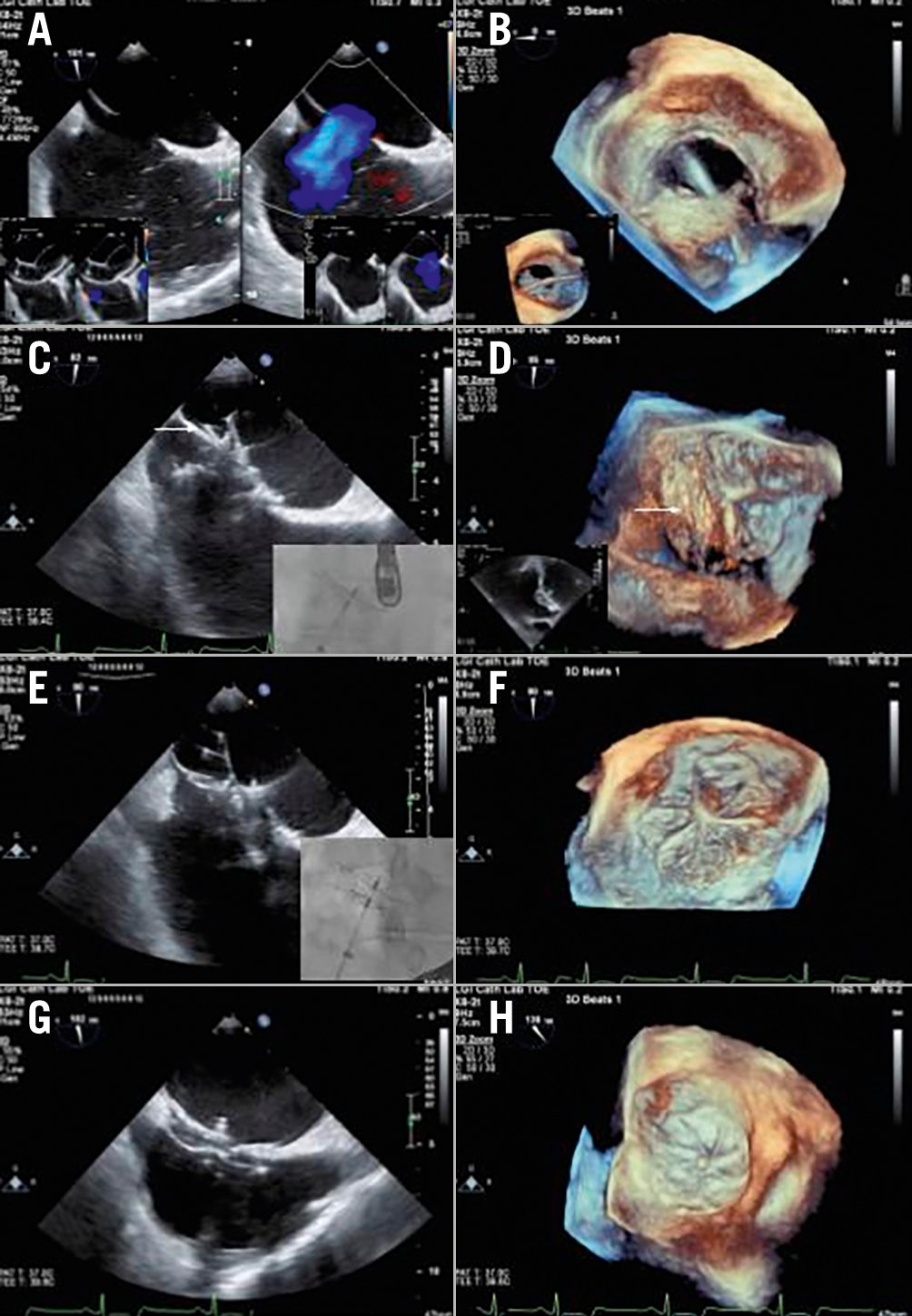
Figure 3. Transoesophageal echocardiography and fluoroscopic images of closure of a complex atrial septal defect using a GORE CARDIOFORM device. A) A 21x18 mm single atrial septal defect (ASD) is shown with left to right atrial low velocity colour flow mapping. It is noted that the primary and secondary septa are separated inferiorly, presenting challenges with encapsulating both septa with the inferior aspect of the disc of the device. The inset figures show balloon sizing stop-flow and deficiency of the ASD to the aorta with a small aortic rim. B) Three-dimensional (3D) left atrial and inset figure right atrial view of the relatively circular ASD. C) A 44 mm GORE CARDIOFORM device has been deployed but the device has failed to capture the inferior septum. This is seen on transoesophageal echocardiography (TOE) (arrow) and also in the inset figure fluoroscopic image which shows that the 2 discs are not separated. D) 3D image of the left atrial disc as in (C), with the arrow tip showing one of the right disc petals in the left atrium. E) Changing to a 48 mm device enabled the inferior aspect of the ASD to be captured with very different and now correct appearances of the device still attached to its delivery catheter and not yet locked both on TOE and also on fluoroscopy (inset image). F) 3D representation of the left atrial disc seen in (E) with none of the right atrial disc seen in the left atrium. G,H) Final appearances of the device following locking in 2 dimensions (G) and 3D (H).
Impact on daily practice
GCA is suitable for a wide range of ASD morphologies and sizes and potentially offers a benefit for the patient due to its softer design, possibly preventing aortic erosion. Device size appears to correlate with the risk of new onset arrhythmias post-procedure. It is possible this could be a reflection of the patient characteristics themselves, rather than device properties. However, device oversizing despite adequate rims has been associated with increased risk of wire frame fractures, and therefore, careful device sizing is recommended. Careful device interrogation both with fluoroscopy (assessing the shape of wire petals) and TOE before and after locking allows the operator to understand whether all rims have been captured and decreases the risk of embolisation. Locking of the device is necessary to understand its final position. Balloon sizing was used and is still in use in all implanting UK centres due to the lack of other available stability checks that can be used with nitinol mesh devices.
Funding
The authors received no sources of funding for this study.
Conflict of interest statement
The authors have no conflicts of interests to declare.

