
Abstract
Aims: To investigate the implantation safety, anatomic performance and septal alignment of the Occlutech® Figulla® Flex occluder (FFO) device, an atrial septal defect (ASD) closure device with specific left-sided deployment characteristics and right-sided septal alignment properties.
Methods and results: Between January 2011 and December 2013 we prospectively collected the change of orientation of the device to the septum during the release process and the feasibility of implantation of the FFO in 122 patients. The mean age was 10.7 years (±10.2), weight 32.9 kg (±20.3), and height 129.4 cm (±30). Devices used were 9 (n=13), 10.5 (n=16), 12 (n=16), 15 (n=39), 18 (n=17), 21 (n=8), 24 (n=5), 27 (n=7) and 30 mm (n=3) in size. No additional implantation techniques were required. Before release, the mean angles of the left and right-sided discs were 29.2° (±9.9°) and 43.4° (±9.2°) to the body axis, and 18.7° (±8.7°) and 27.0° (±10°) immediately thereafter. Thus, there was only a slight change in orientation of the left-sided (10.6°±7.5°) and right-sided (16.3°±7.9°) discs.
Conclusions: The design of this occluder system results in an ideal septum alignment which increases its feasibility as well as patient safety during implantation.
Abbreviations
ACT: activated clotting time
ASD: atrial septal defect
ASO: Amplatzer septal occluder
AV block: atrioventricular block
AV valve: atrioventricular valve
ECHO: echocardiography
FFO: Figulla Flex occluder
Fr: French
IU: international units
OSO: Occlutech septal occluder
PFO: patent foramen ovale
SD: standard deviation
TOE: transoesophageal echocardiography
TTE: transthoracic echocardiography
Introduction
Many procedures have been performed for atrial septal defect (ASD) closure using various devices with different designs, with their respective advantages and disadvantages1. The Amplatzer septal occluder (ASO) (St. Jude Medical, St. Paul, MN, USA) device has been used for about 20 years with good follow-up data, due to its straightforward implantation technique and efficacy in occluding a wide range of ASD sizes2,3. The second generation of the Occlutech device series, the Occlutech® Figulla® Flex septal occluder (FFO) (Occlutech International AB, Helsingborg, Sweden) has new device technology features which seem to be advantageous with regard to feasibility of implantation and device delivery, as well as device alignment to the atrial septum and thereby echocardiographic assessment during implantation.
The aim of this study was to analyse the characteristics of the FFO during implantation, and to describe the septum alignment of the device, the feasibility and success for ASD closure and potential side effects.
Methods
THE DEVICE
The technical details of the FFO as well as the sizes available are described elsewhere in detail4,5. The lack of a left-sided hub creates a lower and more flexible left-sided profile. The device develops in a round, ball-like shape as compared to other devices with a double-sided hub (e.g., ASO) (Figure 1), that configures as a flat disc.
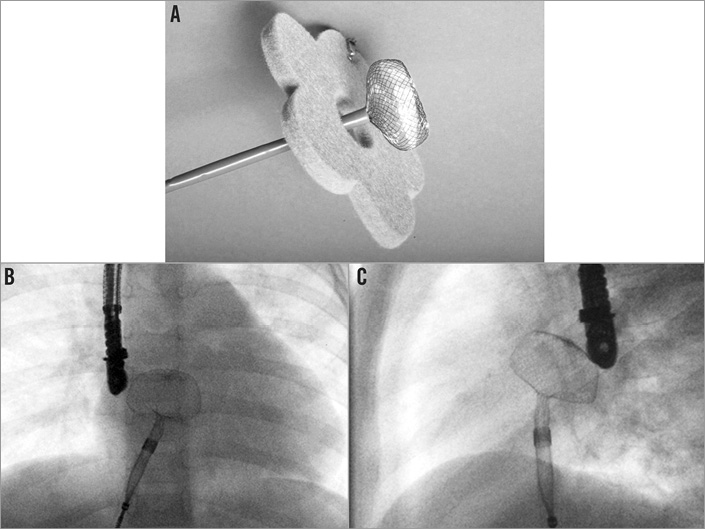
Figure 1. Configuration of the left-sided disc of the Figulla® Flex occluder. A) Development of the left-sided disc of the Figulla® Flex occluder at bench testing. Note the round, ball-like shape of the occluder. B) Development of the left-sided disc of the Figulla® Flex occluder during implantation (a.p. view). C) Development of the left-sided disc of the Figulla® Flex occluder during implantation (lateral view).
In the second generation of this device (i.e., the FFO), the attachment for the delivery system is a ball, connected with a bioptome-like safety system, performing like a ball and socket joint. It allows a tilted angle of 45 degrees without any stress or tension on the implant and improved flexibility for optimal septum alignment (Figure 2). We used the FFO from 2011 onwards for all our ASD closures.
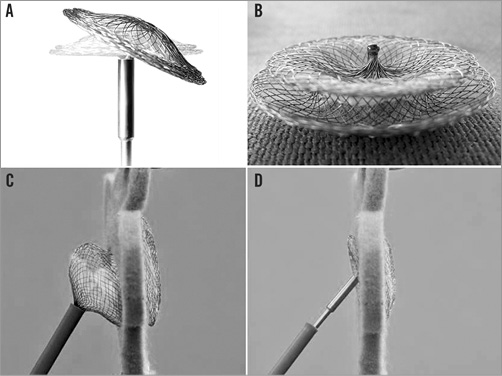
Figure 2. Effect of the specific right-sided connection on the orientation of the device to the atrial septum. A) Documentation of the possible 45° angle of the occluder when attached to the delivery system. B) Documentation of the welded right-sided hub for connection with the delivery cable. C) Deployment of the right-sided disc in an occluder with a conventional screw-mechanism for attachment (bench testing). Note the significant angle and tension between the left-sided and right-sided disc caused by the delivery system. D) Deployment of the right-sided disc in a Figulla® Flex occluder (bench testing). Note that there is nearly no angle and tension between the left-sided and right-sided disc caused by the delivery system.
PATIENT CHARACTERISTICS AND DATA ACQUISITION
All relevant periprocedural data of our consecutive ASD patients from January 2011 until December 2013 were continuously monitored and documented as part of the hospital quality assurance programme. Informed consent was obtained from each patient or the legal guardians. The retrospective analysis was reviewed and approved by the local ethical committee (Ref. no. 64/2013). The angles of the two discs were measured in correlation to the body axis in the lateral plane and documented at the end of every investigation (Figure 3). The results were presented as mean, standard deviation of the mean, or median, wherever suitable. Several subgroups were compared using a Mann-Whitney test (two-sided) due to the small sample size and non-normal distribution.
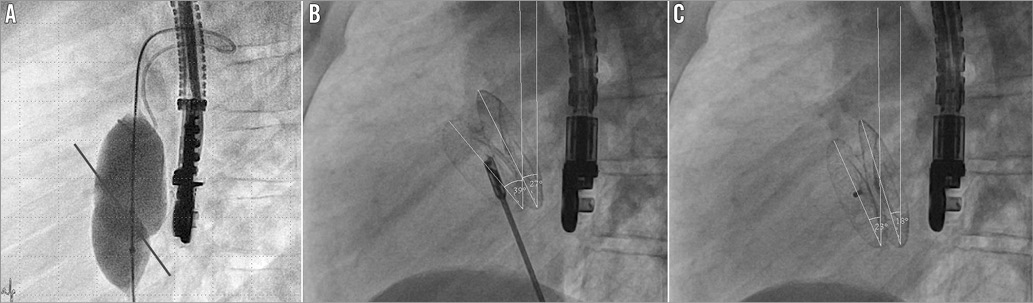
Figure 3. Septum orientation and measurement of the device alignment. A) Balloon sizing of the atrial septum defect (lateral view). The potential axis of the atrial septum is indicated by the painted line. B) After implantation but before release of the occluder the angles between each disc and the body axis are measured (lateral view). In this example there is an angle of 39° for the right-sided disc and an angle of 27° for the left-sided disc. Note that the orientation of the occluder is in congruence with the anatomy assessed by balloon sizing. C) After implantation and complete release of the occluder the angles between each disc and the body axis are measured again (lateral view). In this example (same patient) there is an angle of 23° for the right-sided disc and an angle of 18° for the left-sided disc. Note that there is only a minimal change of the shape and position of the occluder when compared to the pre-release status (B).
IMPLANTATION TECHNIQUE
All interventions were performed under deep conscious sedation using ketamine and propofol and without general anaesthesia6. All ASD closures were performed by registrars or junior fellows as first operators with an experienced consultant supporting. After initial transthoracic echocardiography (TTE), transoesophageal echocardiography (TOE) was performed, the defects were measured with colour Doppler in two planes (i.e., short-axis 10-30° and long-axis 80-110°) to minimise underestimation, the mean was considered as the maximal ECHO diameter, and the presence of an aneurysm or the existence of multiple defects was noted7. Patients were heparinised (100 IU heparin/kg, maximum 5,000 IU); the ACT or any other coagulation parameters were not checked. Routine second-generation cephalosporin was given. Standard balloon sizing was performed with a PTS® sizing balloon (NuMED, Hopkinton, NY, USA) using the minimal waist/stop-flow technique to avoid overstretching the defect. Careful investigation was made to assess the posterior rims regarding stability. In case of a very floppy posterior rim, 1 to 3 mm was added to the defect diameter to calculate the final device diameter. After insertion into the left atrium, the left-sided disc usually configures as a round, ball-like structure (Figure 1). In those cases with a deficient aortic rim, the lateral view is used to guide the assembly to an anterior orientation, allowing an approximation to the posterior part of the aorta. Contact with the septum is confirmed by echocardiography (ECHO) as well as manual palpation. The right side of the device is developed by keeping the device in position. The sheath is withdrawn into the inferior vena cava and the device usually configures well to the septum (Figure 2, Figure 3). Careful echocardiographic evaluation was performed with special focus on the absolute size of the device compared to the atrium, any possible contact with the posterior wall, a firm grip and safe position of the device, especially regarding the posterior and inferior rims, and a possible impairment of the AV valves. The occluder is released after a standard “wiggle” during biplane fluoroscopic documentation. After release, TOE as well as TTE were performed to assess position, residual shunt and procedural success.
DEFICIENT RIMS
Superior and inferior rims were measured by TOE in the long-axis view, and patients with deficient inferior rims (≤4 mm) were excluded as candidates for transcatheter closure. A deficient aortic rim was defined as a distance ≤4 mm, and a naked aorta was defined when no aortic rim was present. These patients were not excluded from implantation.
SEPTUM ALIGNMENT
The angles between each of the two discs and the body axis were measured in the lateral plane, documented at the end of every investigation (Figure 3) and analysed separately before and immediately after release of the device. The differences of angles between the two discs as well as the individual change caused by the release were documented. All the study patients had normal spine axis and no scoliosis.
LARGE DEFECTS
Defects with a stretched diameter greater than 20 mm are usually regarded as large defects8,9. A subgroup analysis of devices with a diameter >20 mm was performed.
SMALL CHILDREN
Since small children as a population often present with a challenging implantation procedure, a subgroup analysis for small children (body weight less than 15 kg) was performed regarding safety and feasibility of ASD closure with the FFO10.
COMPLICATIONS DURING IMPLANTATION AND FOLLOW-UP
Any periprocedural technical difficulties were documented, i.e., additional help to position the device at the left side, cobra-like formation during delivery, multiple efforts for positioning, device embolisation, transient or permanent AV block or perforation, and residual defects by TOE or TTE.
Results
PATIENT CHARACTERISTICS
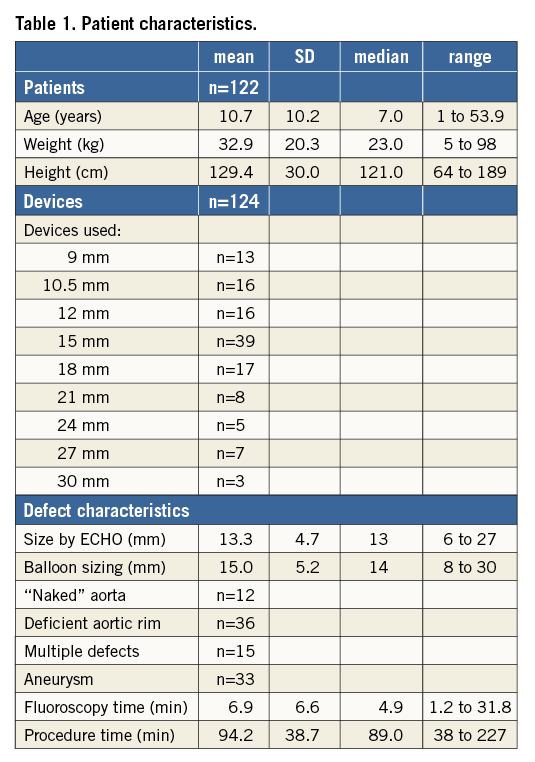
Detailed patient characteristics are shown in Table 1. One hundred and twenty-two patients were treated with the FFO, two of whom received two devices. The mean age was 10.7 years, the mean body weight was 32.9 kg, and the mean body height was 129.4 cm. The devices used were 9 (n=13), 10.5 (n=16), 12 (n=16), 15 (n=39), 18 (n=17), 21 (n=8), 24 (n=5), 27 (n=7) and 30 mm (n=3) in size. Defect closure was performed in two small children without balloon sizing. The mean diameter by ECHO was 13.3 mm, after balloon sizing 15.0 mm. Forty-eight patients showed no or only a deficient aortic rim, 15 patients had multiple defects, and 33 patients had a floppy septum with aneurysm. Interventional defect closure was possible in all patients and without complications. Mean fluoroscopy time was 6.9 min, mean procedure time 94.2 min.
COMPLICATIONS DURING IMPLANTATION AND FOLLOW-UP
There was one embolisation in a 7.6-year-old boy (weight 35 kg, height 130 cm). The defect size was 16 mm by ECHO and 17 mm by balloon sizing, and there was a floppy septum at the inferior-posterior part. We implanted an 18 mm device but, after the wiggle manoeuvre, we did not realise that the device was pushed out at the inferior-posterior edge of the septum into the left atrium. The device embolised into the left ventricle, and during surgery the posterior-inferior rim of the ASD was judged to be extremely floppy over a large proportion of the septum.
There was no occurrence of AV block during implantation or follow-up11 and there were no erosions12. No cobra-like deformation of the larger devices (i.e., size >20 mm) occurred. No supportive techniques for placement of the left atrial disc were necessary, and there was no repetitive “slipping through” the septum even in the large defects. In one patient there was a small residual shunt caused by an additional third small defect close to the atrioventricular (AV) valves. The diameter of this remnant defect was less than 3 mm, and the other defects were adequately covered. In all other patients there was an uneventful follow-up (range six to 46 months, mean 23.4 months, SD±5.9 months).
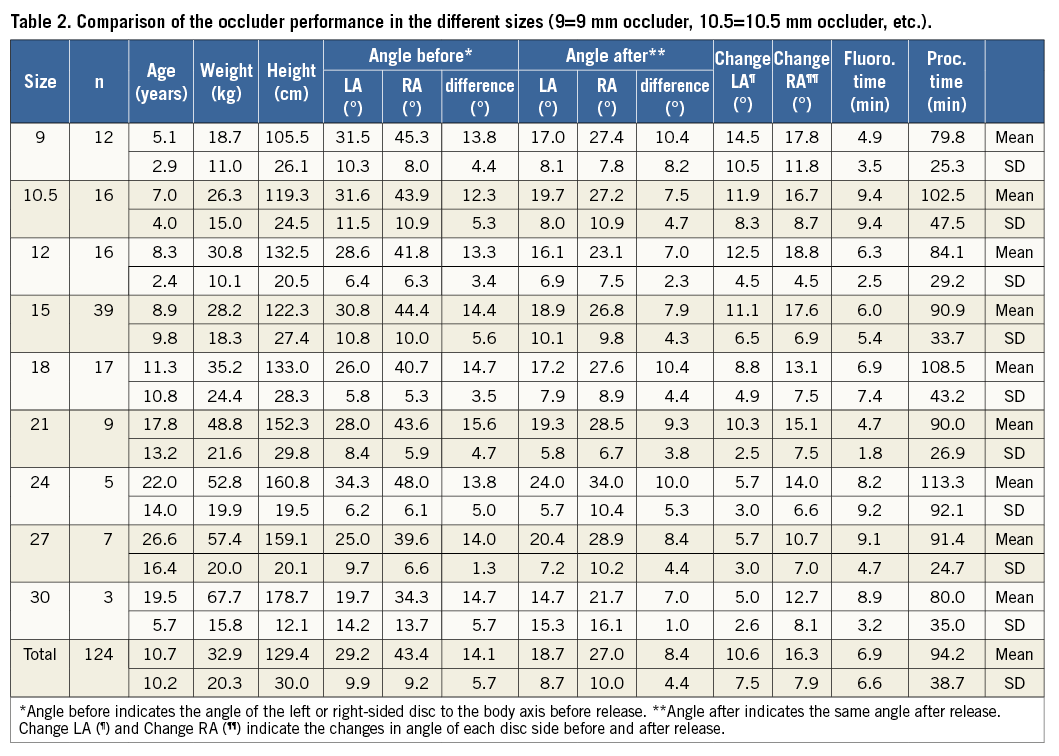
SEPTUM ALIGNMENT ANGLES
The details of the septum alignment angles are shown in Table 2. The mean angle of the right-sided disc to the body axis disc was 43.4° (SD 9.2°, median 43°) before and 27° (SD 10°, median 25°) after release of the device, and the angle of the left-sided disc was 29.2° (SD 9.9°, median 28°) and 18.7° (SD 8.7°, median 18°) before and after release of the device, respectively. The changes of the device during implantation were 16.3° for the right (SD 7.9°, median 17°) and 10.6° (SD 7.6°, median 10°) for the left side of the disc, and the difference in orientation between the two discs was noticeably low, 14.1° before (SD 5.7°, median 14°) and 8.4° (SD 4.4°, median 8°) after release. There is a clear correlation in the size of the patients to the size of the occluders but no difference in septum alignment.
DEFECTS WITH DEFICIENT AORTIC RIMS
There were 12 patients with a “naked” aorta and 36 patients with an aortic rim ≤4 mm, representing a total of 48/122 patients (39.4%) with deficient aortic rims. The demographic data of these patients did not differ from the general patient group. Mean fluoroscopy time in this group was 6.4 min and procedure time 93.7 min (Table 3).
The mean angle of the right-sided disc to the body axis disc was 41.4° before and 24.8° after release of the device, and the angle of the left-sided disc was 26.7° and 16.8° before and after release of the device, respectively. The disc changed by 16.6° for the right and 9.9° for the left side; the difference between the two discs was 14.8° before and 8.0° after release. Therefore, there was no difference in septum alignment or procedural performance detectable (Table 3).
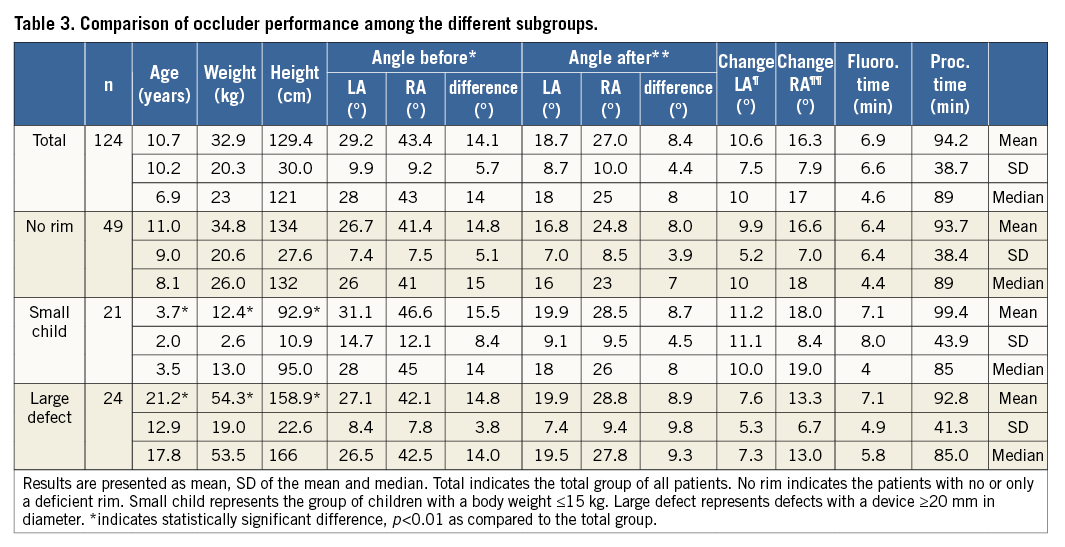
LARGE DEFECTS
Twenty-four patients were treated with a device larger than 20 mm in diameter (19.7%). These patients were considerably older, taller and had a greater weight; however, the implantation characteristics did not differ in a clinically relevant manner (Table 3). The mean age was 21.2 years, the mean body weight was 54.3 kg, and the mean body height was 158.9 cm. The occluders used were 21 (n=9), 24 (n=5), 27 (n=7) and 30 mm (n=3) in size (mean diameter 24.4 mm, SD 3.3 mm), and the mean diameter of the defect by ECHO was 20.5 mm and after balloon sizing 23.2 mm. Sixteen children showed no or only a deficient aortic rim, two patients had multiple defects, and seven patients had a floppy septum and aneurysm. Interventional closure of the ASD was possible in all patients without complications. Mean fluoroscopy time was 7.1 min, mean procedure time 92.8 min. There was one patient in whom we implanted two devices into a floppy septum and in whom fluoroscopy time and procedure time were the longest. The median fluoroscopy time of the group was 5.8 min, and the median of the procedure time was 85 min only. The measured angles of the devices before and after release did not differ at any clinically relevant level as compared to the complete cohort (Table 3).
SMALL CHILDREN
Twenty-one patients presented with a body weight less than or equal to 15 kg (17.2%). These children differed significantly from the general cohort (Table 3). Mean age was 3.7 years, the mean body weight was 12.4 kg, and the mean body height was 92.9 cm. The occluders used were 9 mm in five patients, 10.5 mm in three, 12 mm in two, 15 mm in nine and 18 mm in two patients (mean diameter 12.9 mm, SD 3.0 mm, median 15 mm). Defect closure was performed in two patients without balloon sizing. The mean diameter by ECHO was 10.3 mm, and after balloon sizing 12.3 mm. Six children showed no or only a deficient aortic rim, three patients had multiple defects, and eight patients had a floppy septum with aneurysm. Device closure was possible in all patients without complications. Mean fluoroscopy time was 7.1 min, and mean procedure time 99.4 min. There was one patient who required additional evaluation of pulmonary hypertension as well as patent ductus arteriosus (PDA) closure in whom fluoroscopy time and procedure time were the longest. The measured angles of the devices before and after release did not differ at any clinically relevant level as compared to the complete cohort (Table 3).
Discussion
This is the first case series analysing the specific device properties of the FFO. Mortezaiean et al most recently reported its successful use in 45 children13 and Froehle et al reported the use of a large FFO to close a gigantic PDA in a 35-year-old woman14.
ORIENTATION OF THE LEFT-SIDED DISC
By avoiding a left-sided hub, flexibility is increased and, most importantly, the left atrial disc develops in a round, ball-like shape (Figure 1) but not as a flat disc, which might pull the device diagonally across the septum and “slip through the ASD” in a considerable number of cases15.
To overcome this problem, many different techniques have been described, namely the use of additional wires, balloon-assisted techniques, specific sheaths, deployment of the ASO device in pulmonary veins, etc15. In our case series, none of these techniques was necessary nor was a slipping through the defect noted, despite the fact that most of the procedures were performed by relatively inexperienced operators and in a notably high number of patients with deficient aortic rims or large ASDs. We believe that this may be due to the beneficial shape of the device during left atrial deployment.
ORIENTATION OF THE RIGHT-SIDED DISC
When using commonly designed closure devices, the atrial septum very often becomes distorted by the tension of the delivery cable, and correct judgement of residual shunt becomes difficult. After release, the atrial septum returns to its natural position and the device typically moves (“jumps”) superiorly and leftwards. Only then can the final result be estimated correctly15. The right-sided disc of the FFO is angled up to 45° without significant tension during implantation. These minimal changes and, more importantly, the negligible difference in orientation between the two discs reflect the trivial change in orientation and the almost ideal septum alignment. The size of the device, the presence of deficient aortic rims or the use in small children has practically no impact on the septum alignment during implantation (Table 3).
PROCEDURE TIME
Despite junior operators, the overall procedure (mean 94.2 min, SD 38.7 min, median 89 min) and fluoroscopy (mean 6.9 min, SD 6.6 min, median 4.6 min) times were notably short as compared to other reports where fluoroscopy times exceeding a mean of 10 min have been reported1,2. This may be an indication for the feasibility, simplicity and thereby safety of device implantation.
SMALL CHILDREN
Our proportion of 21/122 (17.2%) small children is comparable to the multicentre series presented by Cardenas et al who reported on 52 children out of 484 patients (10.7%) with minor complications in eight patients (15.4%)10. Similar rates of complications have been reported by others. None of these complications occurred in our subgroup of small children. The procedure time as well as fluoroscopy time in our patients were considerably less as compared to those reported elsewhere.
LARGE DEFECTS
Of the 124 occluders used, 24 (20%) were larger than 20 mm in diameter with a mean device diameter of 24 mm. This represents an adequate incidence of larger defects in paediatric patients with complex anatomy as compared to other reports where the incidence varies between 9% and 32%. It may even represent up to 45% when the majority of patients are recruited from adult cohorts1,8. ASD closure was successful in all patients without additional fluoroscopy or procedure time.
SIDE EFFECTS
EROSIONS
Even with a high rate of deficient aortic rims (48/122, 40%), we did not see any erosions12. It may be speculated that the design may increase flexibility and therefore reduce the shear forces as compared to the ASO; however, the number of patients treated as well as the follow-up time are far too small to allow any reasonable conclusion on this phenomenon.
AV BLOCK
We did not encounter any transient or permanent arrhythmias, and in particular no occurrence of AV block. As part of our safety strategy, we excluded patients with an unfavourable device to patient size ratio (i.e., device size in mm greater than patient weight in kg).
EMBOLISATIONS
We encountered only one embolisation (0.8%) in a patient with a very floppy posterior-inferior rim. This embolisation can be accounted for as operator-related.
Study limitations
This single-centre study was not conducted with a prospective study design or as a controlled study comparing the FFO to the ASO. However, the information presented was collected in a prospective manner in consecutive patients according to the hospital’s policy. In the same way, side effects, problems during implantation and immediate follow-up were monitored. Therefore, the possibility of inadequate data acquisition seems extremely low.
We acknowledge that, due to projection artefacts and individual anatomy of the atrial septum compared to the body axis, there is a chance of a systemic mistake in the judgement of the occluder position and this method of measuring the angles of the two discs in relation to the body axis has some limitations. Due to the lack of other comparable methods, we think that this approach might be the most objective way of describing the configurational changes of the occluder and its alignment to the atrial septum.
The total number of patients may be judged as inadequately low; however, many single-centre reports of similar case series present data of fewer than 100 patients over a comparable time period. The age range, and the different sizes and anatomies of the defects presented seem representative for a normal patient cohort for interventional ASD closure.
Conclusion
The lack of a left-sided hub enables the FFO to develop in a round shape during left atrial deployment, minimising the risk of the device prolapsing across the atrial septum and eliminating the need for additional support to stabilise and deploy the device. The specific mobile attachment to the delivery cable leads to an improved septum alignment even in small children, large ASDs or in defects with a deficient aortic rim without significant tension or distortion of the discs and only minimal change of orientation or shape after complete release.
| Impact on daily practice The FFO has specific design properties which minimise the risk of the device prolapsing across the atrial septum and eliminate the need for additional support to stabilise and deploy the device during ASD closure. The specific mobile attachment to the delivery cable leads to an improved septum alignment even in small children, large ASDs or in defects with a deficient aortic rim without significant tension or distortion of the discs and only minimal change of orientation or shape after complete release. Based on the properties described, we believe that this occluder system is a significant improvement for the interventional closure of atrial septal defects regarding feasibility as well as patient safety during implantation. |
Funding
The funding is by hospital funding only.
Conflict of interest statement
The authors have no conflicts of interest to declare.

