Background
Both, patent foramen ovale (PFO) and atrial septal defects (ASD) are openings in the interatrial septum, but differ with respect to morphology and functional consequences. Accordingly, although technical treatment details match in several aspects, treatment indications vary. The foramen ovale, a valve-like opening of the interatrial septum during intrauterine life closes usually in the first months after birth, but remains permanently open in around 30% of the population. ASDs represent the second most common congenital heart defect, accounting for about 10% of all congenital heart defects in the adult population. Depending on the localisation in the atrial septum, ASDs are basically divided into four different types. All of them have similar haemodynamic consequences, however, only the secundum ASD, which is the most frequent one and located in the central portion of the interatrial septum, can be treated interventionally and will be discussed in this paper.
Indications
At present, the best established indication for closure of PFOs is secondary stroke prevention in patients without additional potential causes for an embolic event (cryptogenic stroke). PFO closure is indicated in divers with risk of decompression sickness and patients with various forms of hypoxia/hypoxaemia syndromes. In addition, migraine (with aura) as another potential indication is currently being discussed, and studied based on reports indicating a link between migraine and PFOs.
Apart from the potential for paradoxical embolism, like in patients with PFO, the principal indication for ASD closure is the haemodynamic overload due to a significant left-to-right shunt, resulting in stretching of atrial and right ventricular walls and finally atrial arrhythmias, myocardial failure and pulmonary hypertension.
Methods
Before the procedure every patient should undergo a profound morphology assessment using transesophageal echo (TEE). The implant procedure can be performed under local anaesthesia using fluoroscopic guidance only, but additional echographic imaging is common, particularly for closure of large ASD’s. After access through the femoral vein, the defect is crossed under fluoroscopic guidance using a guidewire, or with the help of a 5 Fr or 6 Fr multi-purpose or similarly shaped catheter. For ASD closure, balloon-sizing is recommendable to determine the defect size, as echography tends to underestimate the true size, particularly in the presence of floppy portions of the adjacent septum. The Amplatzer device family for PFO and ASD closure is most commonly used. Although there are various alternative devices available, particularly in the PFO market (Figure 1). In PFO closure using a double disk Amplatzer device, the left atrial disk is developed in the left atrium, device and sheath are then pulled back as a unit against the atrial septum (Figure 2). In contrast, for ASD closure, additional deployment of the neck and a small part of the right atrial disk in the left atrium ensures that the device is pulled through the centre of the defect (Figure 3). Once the left disk is in close contact with the septum, the right atrial disk is deployed completely in the right atrium by retracting the sheath. After confirmation of an adequate position by echocardiography or right atrial contrast angiography with following the contrast medium through to the levophase depicting the left atrium the device is released by unscrewing. Angiography needs to be done in a profile projection, completely separating the 2 disks.
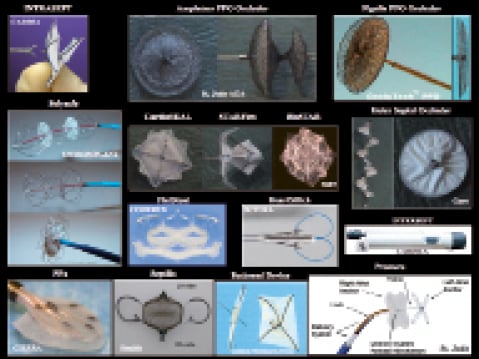
Figure 1. Examples of PFO closure devices.
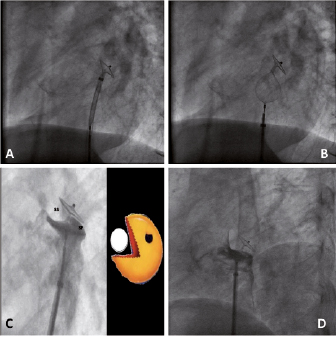
Figure 2. PFO closure using an Amplatzer PFO occluder. A. Left disk developed in the left atrium, contacting the septum after withdrawal, indicated by a clear change of disk orientation. B.Deployment of the right disk in the right atrium with continued tension on the system, keeping the left disk in place. C.Angiographic “pacman” sign, indicating a correct device position – due to the thick muscular septum secundum (ss) and the membranous septum primum (sp), the disks are not in a parallel position if properly placed (V-shape).
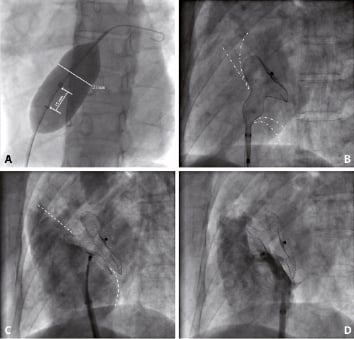
Figure 3. ASD closure using an 36mm Amplatzer ASD occluder. A.Balloon-sizing, revealing a waist with a diameter of 31mm. B.Deployment of left disk as well as parts of the right disk to centre the device. C.Development of the complete right disk in the right atrium. D.Final contrast injection confirming correct device placement.
Adjunctive medication includes periprocedural anticoagulation with heparin and administration of antibiotics followed by short-term double antithrombotic therapy (clopidogrel for one to three months, acetylsalicylic acid for at least five months if not permanently indicated for other reasons). Transthoracic echocardiography should be performed before discharge to confirm the correct device position and should be repeated at six months post procedure to exclude residual shunts, device failure, or thrombus attachments.
Difficulties
Complications of ASD/PFO closure depend on experience of the operator, type of device used, and characteristics of the defect. The risk of embolisation is practically inexistent with PFO closure in experienced hands. In ASD closure, there is some risk for embolisation, particularly in cases with device-defect mismatch. Embolisation occurs usually immediately after deployment. Depending on the site of embolization and site of device, the device can often be retrieved without need for surgery (e.g. embolisation to pulmonary artery). Embolisation to the aorta may need a cut-down in the groin for final device removal.
Late complications such as device thrombosis occur in less than 1% and very few clinical sequelae have been reported. Erosion of a free wall of the atrium, most likely where it pounds against the pulsating aorta has been reported but it is exceedingly rare and inexistent with small devices not reaching the aorta. Air embolism, infections or blockages of inflow from pulmonary veins or the coronary sinus are other theoretical complications hardly seen in clinical practice. Transient palpitations are common. Atrial fibrillation can occur acutely or in the follow-up period and should be managed following standard practice.
Based on the excellent closure rates and low incidence of complications with current devices, PFOs and suitable ASDs are closed interventionally rather than surgically. Decision making is not always as straight-forward. Small ASDs without dilatation of the right atrium and right ventricle and a Qp:Qs <1.5:1 were not approached while surgery was the only option. Interventional closure of a small ASD, on the other hand, is an extremely safe outpatient procedure. Hence, the dictum appeals: large ASDs should be closed because they are large, small ASDs should be closed because they are small. Multiple ASDs (cribriform variety) or large ASDs with small or absent rims are not, per se, acontraindication for device closure, but success rates are reduced and surgery will finally be necessary in a significant percentage of them.
Conflict of interest statement
Bernhard Meier has received research grants and advisor fees from St-Jude-AGA Medical. The other authors have no conflict of interest to declare.

