
Abstract
Aims: Previous comparisons between AMPLATZER septal occluders and other designs were retrospective, non-randomised, non-concurrent and involved fewer patients. A prospective concurrent head-to-head comparison of AMPLATZER (ASO), Cera (CSO) and Figulla (FSO) septal occluders was planned to study the patient outcomes.
Methods and results: The three occluders were serially allocated in a cycle of three to consecutively included patients. Demographic, procedural details and complications were analysed. After calculating a sample size of 122 patients in each group, additional patients were recruited to ensure at least 80% follow-up. Four hundred and fifty (450) consecutive patients equally divided among the three designs were comparable in all parameters. There were no major complications and procedural success was 99.6%. The defects and device sizes were similar in all groups; the delivery system was significantly smaller with the ASO. The FSO needed special deployment techniques less often and formed a cobra deformity more often, though this was not statistically significant. Patient outcome was similar among the groups at a follow-up of 12-47 months.
Conclusions: The new occluders are comparable to the ASO with good outcomes and low complication rates in the current era. The new modified structural designs do not show any advantages in terms of procedural complications on early and midterm follow-up, but long-term studies are warranted.
Introduction
Transcatheter closure is increasingly adopted for treating secundum atrial septal defects (ASD). Avoidance of scar, shortened hospitalisation, reduced bleeding, arrhythmia and post-pericardiotomy syndrome make them an excellent alternative to surgery1. The occluders differ in their properties, materials, flexibility and deliverability and are available in different sizes2. The AMPLATZER™ Septal Occluder (ASO; St. Jude Medical, St. Paul, MN, USA) is the prototype of the braided nitinol self-centring double disc device, approved worldwide for the past two decades3. Recently, a few other occluder designs with structural innovations such as the Cera™ Septal Occluder (CSO; Lifetech Scientific, Shenzhen, China) and the Figulla® Septal Occluder (FSO; Occlutech, Helsingborg, Sweden) have been developed and successfully used.
Small trials compared these occluders, but they were implanted in different time frames involving differing operator learning curves2,4,5,6. None of these studies randomised the occluders. Most trials compared only two designs at a time4,5,6. A head-to-head concurrent comparison of these three designs in consecutive real-world patients will ideally study their safety and outcome data. Performance of all procedures under the supervision of a single operator will render procedural uniformity. A blinded study is not feasible as the occluders differ significantly in their appearance, delivery techniques and available sizes.
Materials and methods
INCLUSIONS AND EXCLUSIONS
Criteria for inclusion were (1) haemodynamically significant shunt ratio more than 1.5 with right ventricular enlargement or symptoms related to the shunt, (2) defect size on echocardiography less than 38 mm, (3) presence of at least 5 mm margin on all sides except retro-aortic margin (the decision to include a patient with either a deficient posterior or inferior margin between 3-5 mm was taken by the principal operator after considering the adequacy of the adjacent confluent margins), (4) multiple defects (one large or two sandwiched devices were likely to stop most of the flows), and (5) indexed pulmonary vascular resistance less than 8 Wood units. Additional cardiac lesions requiring surgical correction, multiple defects that could not be adequately closed by one or multiple devices, significant coronary atherosclerosis, nickel allergy and contraindications to antiplatelet therapy were exclusions.
PATIENT SELECTION AND ETHICS APPROVAL
Patients eligible for device closure were enrolled in the comparative study. All patients or their guardians individually consented for the use of different devices and participation in the study. The institutional review board permitted this prospective head-to-head comparative trial with different devices. The principal investigator monitored patient selection and performance of all procedures. Assessment with transthoracic echocardiography (TTE) was the routine in most patients, especially young children. Use of transoesophageal echocardiography (TEE) was restricted to adults, patients with poor transthoracic images, multiple defects needing single or double devices and defects larger than 30 mm. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
DEFECT DIAMETER AND DEVICE SELECTION
The largest echocardiographic defect diameter formed the basis of choosing the device size. The device size was chosen 2 mm larger in children, 4 mm in adults, and 4-6 mm in defects more than 30 mm with floppy margins. Balloon sizing of the defect was performed only in multiple defects that required two devices, where two sizing balloons were simultaneously inflated to measure the stop flow diameter in both the defects. A Mullins sheath (Cook Medical, Bloomington, IN, USA), one size larger than that recommended for the selected device, was chosen for device delivery.
OCCLUDER DESIGNS
All three occluders are repositionable, double disc nitinol wire-mesh systems with a waist that self-centres on the defect. The CSO is a more flexible wire-mesh system coated with titanium nitride to enhance smooth endothelialisation and minimise nickel leaching7. The FSO has a titanium oxide coating to reduce nickel leaching and has no hub on the left atrial surface in order to reduce trauma and clot formation; its flexible cable allows full circular movement and angulation4,8.
DEVICE SIZES
The ASO is available in 27 different sizes ranging from 4-40 mm with 1 mm increments up to 20 mm and 2 mm thereafter3. The CSO is available in 18 different sizes from 6-40 mm with 2 mm increments7. The left disc of the ASO and CSO measures 12 mm more than the waist in devices smaller than 10 mm, 14 mm more in devices up to 32 mm and 16 mm more in larger devices. The right disc measures 4 mm less than the left disc in devices up to size 32 mm, and 6 mm less in larger sizes. The FSO is available in 15 sizes from 6-40 mm, at increments of 1.5 mm till 12 mm and 3 mm thereafter. The left and right discs of the FSO are 10.5-16 mm and 6.5-10 mm larger than the waist, respectively4. The CSO and FSO have fewer size options compared to the ASO.
SERIAL ALLOCATION TO DIFFERENT GROUPS
The different occluder designs, namely ASO, CSO and FSO, were serially allocated to the included patients in consecutive order, one after the other, in a cycle of three. This method of allocation of patients was followed in order to avoid an unequal number of subjects in each group; therefore, random numbers were not used. As the study patients were serially enrolled on a continuous all-comer basis, stratified randomisation was also not feasible.
PROCEDURE
All patients received pre-treatment with one dose of aspirin, preprocedural antibiotics and heparinisation after vascular access. Shunt ratio and pulmonary vascular resistance were calculated from oximetry and haemodynamic recording. A coronary angiogram was performed in patients above 40 years and those with atherosclerotic risk factors. When conventional deployment failed, special techniques such as the pulmonary vein (left or right upper) deployment technique or balloon-assisted techniques were used9. In case of patients requiring a second device for an additional defect, two femoral venous accesses were used and the larger device sandwiched the smaller device.
FOLLOW-UP
Clinical evaluation, electrocardiography and TTE (to ascertain the position of the device, residual shunt and device-related complications) were performed before discharge from hospital at one-, three-, and six-month follow-up and yearly thereafter. Oral aspirin with or without clopidogrel (for devices larger than 30 mm) was prescribed for six months. A telephonic contact was made with patients who did not attend the follow-up clinic.
COMPLICATIONS
Major and minor complications were defined in accordance with the previous guidelines10. Major complications included events that are life-threatening, prolong hospitalisation, have long-term consequences or need for ongoing therapy such as stroke, thromboembolism, erosion or pericardial effusion with tamponade, arrhythmias requiring anti-arrhythmics or pacemakers, device embolisation requiring surgical removal and death. Minor complications included non-life-threatening events which neither prolonged hospitalisation nor required surgery such as device embolisation with percutaneous retrieval, cardiac arrhythmia without prolonged need for medications, vascular access-site complications, pericardial effusion managed medically and thrombus formation without embolisation. Success was defined as occlusion of the atrial septal defect without any significant residual shunt10.
SAMPLE SIZE
The previous smaller comparative trials observed statistically significant differences only in the size of the delivery systems without any major differences in adverse events4,5,6. This study was powered based on the primary endpoint of these studies. Using a one-sided type I error of 2.5%, 122 patients were required in each group to provide 90% power. We enrolled 150 patients in each group, assuming that at least 80% would be available for the entire follow-up period.
STATISTICAL ANALYSIS
Statistical analysis included demographic (age, sex, weight, body surface area), echocardiographic (maximum defect diameter, adequacy of rims), procedure-related (procedural time, fluoroscopy time, final device size, size of delivery system, additional deployment techniques, number of attempts), haemodynamic (shunt ratio, pulmonary artery pressures) and outcome (procedural success, residual shunts if any, complications at discharge and during follow-up) parameters. Continuous variables were compared using the one-way ANOVA test. Categorical variables were compared using the chi-square test or Fisher’s exact test. SPSS Statistics Software, Version 23 (IBM Corp., Armonk, NY, USA) was used.
Results
In this single institution study from August 2014 to July 2017, 450 consecutively included patients were serially assigned and equally divided among the three occluders. In the same time period, 141 excluded patients underwent surgery. The procedure failed in two (0.4%) patients. One 18-year-old male with a deficient retro-aortic margin and thin floppy postero-inferior margin in the ASO group was sent for surgery as the device repeatedly failed to deploy. Another 38-year-old female with multi-fenestrated ASD closed with two FSO devices had a small haemodynamically insignificant residual shunt of 6 mm between the two devices, which persisted on follow-up.
COMPARISON OF CLINICAL CHARACTERISTICS
Table 1 shows a comparison of the demographic, echocardiographic, haemodynamic, procedural and follow-up data among the three groups. There were no statistically significant differences in demographic and procedural parameters. Females accounted for 66% of the study population; 47% were under 18 years of age. Thirty-seven percent (37%) of the patients had pulmonary hypertension defined as mean pulmonary artery pressure above 25 mmHg.
COMPARISON OF DEVICE SIZE
There were no significant differences in defect diameter, device size and device-defect ratio. There were no differences in the number of patients with inadequate margins among the groups. Post hoc analysis showed both CSO (p<0.001) and FSO (p=0.007) needing significantly larger sheaths compared to the ASO, but the FSO and CSO did not differ significantly between themselves (p=0.21).
COMPARISON OF DEVICE DEPLOYMENT
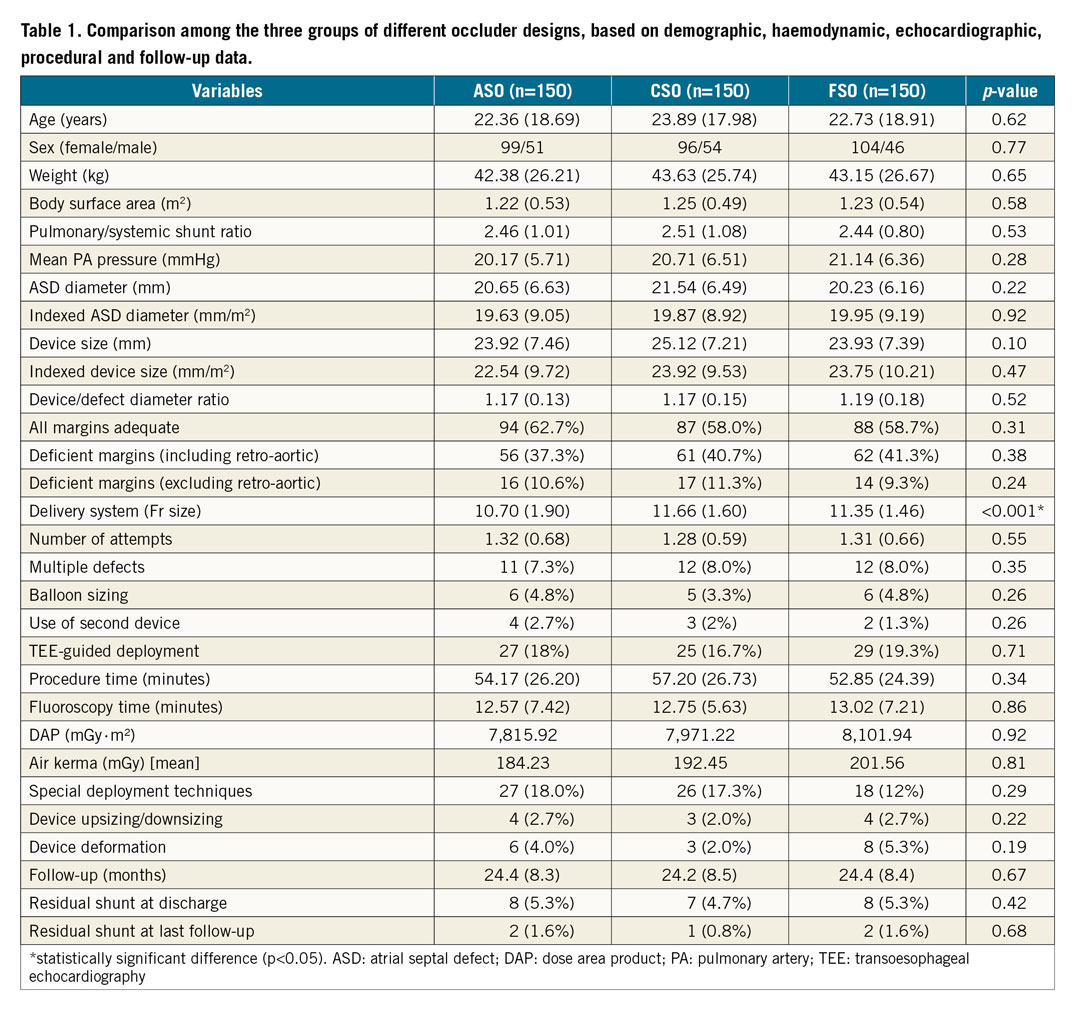
Four patients in the ASO group, three in the CSO group and two in the FSO group required a second device for closure of additional defects. Need for balloon sizing of the defect, need for TEE guidance, and number of attempts at deployment were not significantly different. Special deployment techniques were needed in 27, 26 and 18 patients of the ASO, CSO and FSO groups, respectively; this was not significant. Eleven patients required either upsizing or downsizing of the device; this was similar among the groups. A cobra deformity occurred in a total of 17 cases; this did not vary significantly among the three groups (ASO=6, CSO=3, FSO=8, p=0.19) (Figure 1). The procedure time was similar among the groups.
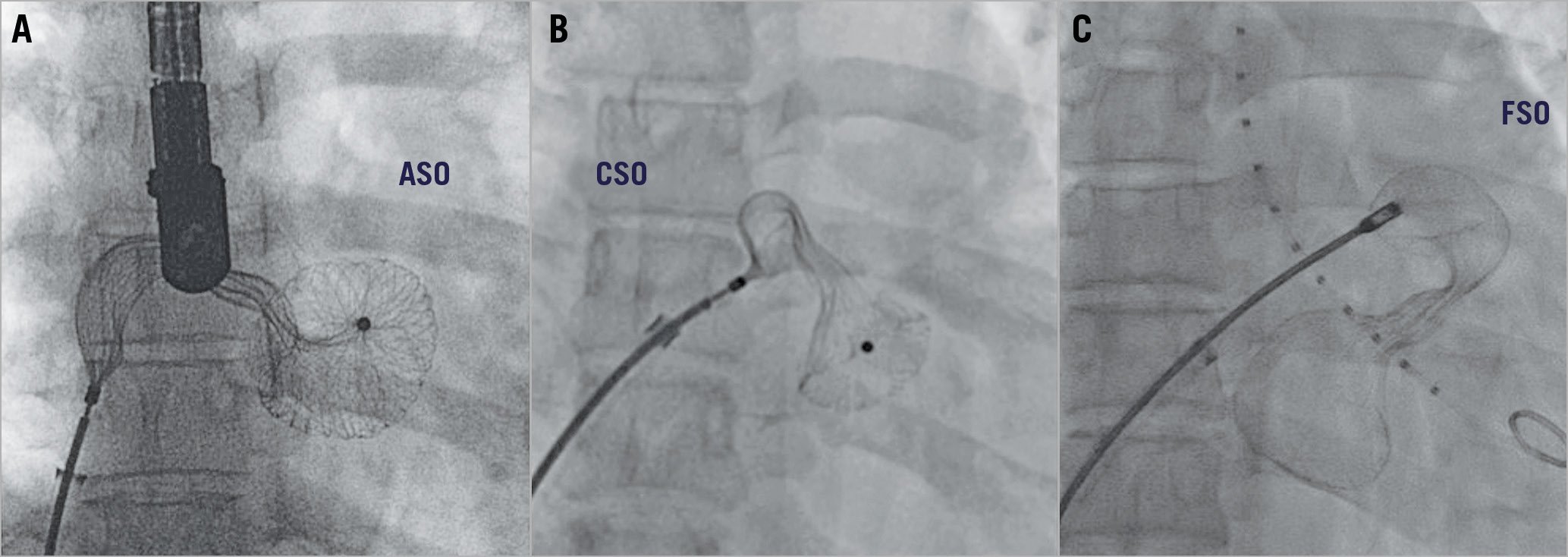
Figure 1. Cobra formation. All of the cobra formation memory abnormalities of the AMPLATZER Septal Occluder (A), the Cera Septal Occluder (B) and the Figulla Septal Occluder (C) were successfully managed in the catheterisation laboratory by manipulating the device within the atrium to enable the devices to retain their memory.
COMPLICATIONS
There were no deaths, perforations or erosions, tamponade, major vascular complications, surgical device retrieval or any other major complications in the study (Supplementary Table 1). Minor complications included brief atrial tachyarrhythmias (n=10, 2.2%), post-procedural fever (n=10, 2.2%), mild pericardial effusion (n=10, 2.2%), air embolism (n=12, 2.7%), transient atrioventricular blocks (n=10, 2.2%), minor vascular complications (n=7, 1.5%), successfully retrieved device embolisation (n=2, 0.4%), deep vein thrombosis (n=1, 0.2%), kinking of delivery sheath (n=1, 0.2%) and transient ischaemic attack (n=1, 0.2%). There was no significant difference in complications among the three groups (ASO - 23 [15.3%], CSO - 21 [14%], FSO - 20 [13.3%], p=0.54). Among five patients with preprocedural atrial fibrillation, two were electrically cardioverted and one medically cardioverted with amiodarone. The others were treated with anticoagulation.
DEVICE EMBOLISATION
There were two device embolisations. A 44-year-old lady with two balloon-sized defects measuring 28 mm and 20 mm had embolisation of both 30 mm and 22 mm ASO devices into the left ventricle after deployment. Both the devices were snared successfully, and the defects were closed with larger devices (Figure 2). Another 6-year-old girl with an 18 mm defect with a floppy postero-inferior margin had embolisation of a 20 mm CSO device into the left atrium that was subsequently snared and upsized to a 24 mm device.
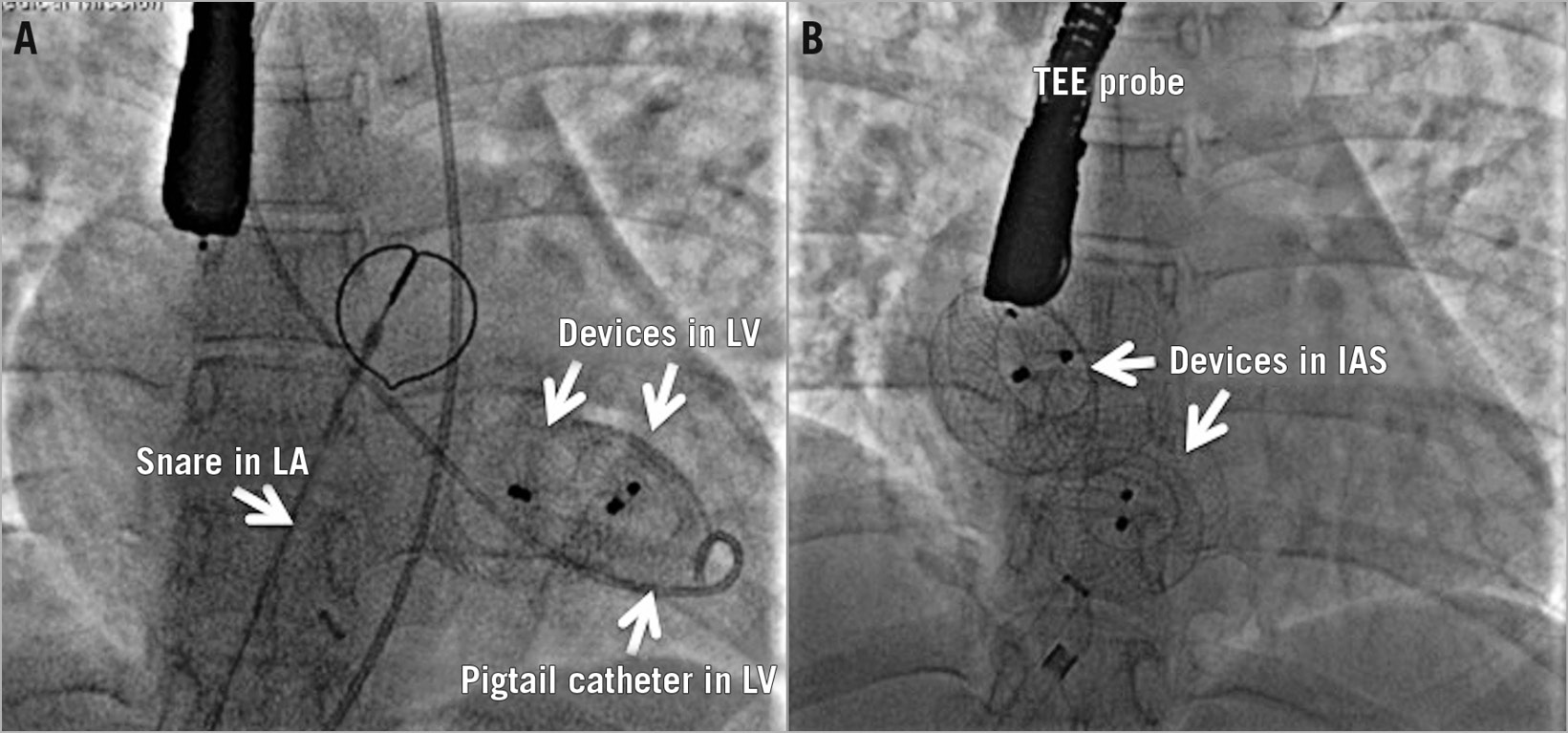
Figure 2. Embolisation. Two AMPLATZER Septal Occluder devices deployed across two large atrial septal defects embolised after release into the left ventricle (A). Both devices were displaced from the left ventricle with a pigtail arterial catheter into the left atrium, successfully snared and replaced with larger devices (B).
FOLLOW-UP
Insignificant residual flows in the pre-discharge echocardiogram in 23 patients (5.1%) were not different in the three groups (ASO - 8, CSO - 7, FSO - 8). The flows disappeared in almost all patients on a median follow-up of 32 months (12-47 months). Classification of residual shunt as trivial, mild, moderate and severe was not carried out, as the shunt was not significant in all except one patient. None of the patients presented with new significant pericardial effusion, new-onset arrhythmia, stroke, cardiac perforation, device erosion, or embolisation during the follow-up.
Discussion
The ASO is the most widely used ASD closure device with more than 300,000 implants across the world1. Despite its commendable safety, efficacy and excellent follow-up outcomes, cardiac erosions described in 0.1-0.3% of patients concern the implanting physicians. With no absolute risk factor for erosions, multiple relative risk factors are postulated11. As the delivery cable of the ASO is not always perpendicular to the plane of the interatrial septum, sudden jump during device release may lead to embolisation12. Coated nitinol wires reduce thrombogenicity and minimise nickel leaching; an angulated delivery pusher avoids undue tension on the device. The FSO and CSO have shown equal safety and efficacy in small comparative trials (Supplementary Table 2) in immediate and midterm follow-up4,5,6. However, no head-to-head comparison included all three devices. Similar implantation techniques ensure that there is no additional learning curve involved. Our prospective concurrent head-to-head comparative study analyses the procedural outcomes in patients with similar demographic parameters.
DEFECT SIZE
The defect and device sizes were not statistically different among the groups. A limited choice of occluder sizes in the FSO led to a larger device size in a previous small trial4. Our study with a larger number of patients failed to observe such difference. While balloon sizing was routine in the past, recent investigators used maximal echocardiographic diameter or colour-flow diameter4. We too used the echocardiographic diameter. Balloon sizing prolongs the procedure and increases costs4.
DELIVERY SHEATH SIZE
The delivery sheath size differed significantly among the groups. It was smaller in the ASO. The need for a larger introducer sheath is mentioned as a limitation of the FSO and CSO6,13. However, this did not translate to higher vascular access complications in our study or in any of the previous studies. Nevertheless, this may influence device choice in younger patients.
PROCEDURAL EASE, DURATION
Short fluoroscopic time with use of the FSO compared to the ASO observed in a previous smaller trial was attributed to ease of deployment provided by the tilt-rotatable delivery system and better conformability of the FSO4. There was a non-significant lower requirement for special deployment techniques for the FSO (12%), compared to the CSO (17.3%) and the ASO (18%) in our study. However, there were no differences in procedural and fluoroscopic time or the radiation dose6. The use of a second device for closing an additional defect in the same patient was similar in the groups, indicating the suitability of all these devices in multiple defects. No previous study has compared the need for special deployment techniques and second device deployment amongst the devices.
MAJOR COMPLICATIONS
Two procedural failures in this study with a resultant success rate of 99.6% reflected the excellent procedural outcomes4,5,6,13,14. There were no major complications, again indicating the maturity of the learning curve. Operator experience aids proper patient and device selection and special manoeuvres to succeed in all procedures. However, long-term follow-up is required to look for delayed complications such as erosions or thrombosis related to the use of large devices. Comparisons in the past also failed to detect any difference in major complications among the different occluders2,4,5,6,13,14.
ARRHYTHMIAS
There were a few minor complications in each of the groups; however, these did not vary significantly among the groups. Periprocedural atrial tachyarrhythmias occurred in 2.2% of patients, were similar in all groups and none required prolonged treatment. Arrhythmias were attributed to inflammatory reactions as well as stretching of the atrial septum15. Even though a flexible FSO with a smaller disc size was proposed to reduce their incidence, our study failed to identify any difference4. Transient atrioventricular nodal conduction block, seen in 2.2% of our group and which recovered spontaneously in all, was also noted in 1.72% of patients in a previous report16.
DEVICE EMBOLISATION
Embolisation of the device in 0.4% of patients in our study was marginally lower than the 0-3% in previous studies4,6,17,18,19,20. Both of the embolisations (one each of the ASO and CSO) were successfully retrieved and replaced by larger devices (Figure 2). Embolisations are often related to the anatomical features such as floppy or deficient margin; a careful device selection prevents this. As the delivery cable is non-tilting and perpendicular in the ASO and CSO, a jump during the device release could predispose to embolisation of an unstable device12.
OTHER COMPLICATIONS
Transient ST elevation due to air embolism, mild pericardial effusion, post-procedural fever, access-site haematomas, deep vein thrombosis, and transient ischaemic attacks in our study were within the range reported in the previous studies. There were no differences among the groups. Despite the differences in the stiffness of the wires, presence or absence of left atrial disc hub or coating to minimise nickel leaching, our study did not show any clot formation on the left disc, embolic manifestations, trauma to the left atrium or nickel allergy in any of the groups. Routine use of heparin and aspirin might have curtailed thrombogenicity16. Long-term follow-up studies are required to confirm such a lack of differences.
DEVICE NITINOL MEMORY
Cobra deformations are memory abnormalities21. In our study, cobra formation was more frequent with the FSO (5.3%) than the ASO (4%) and the CSO (2%), although this was not statistically significant (Figure 1). However, these were managed successfully in the laboratory. Excessive twisting of the wires in the waist segment of the device has been suggested for the frequent occurrence of cobra deformity with the FSO21.
RESIDUAL SHUNT
Immediate post-procedural residual shunt was reported to be more common in the FSO compared to the ASO in one study, attributed to a smaller right atrial disc and altered thrombogenicity of the polyester patch6. However, the residual shunts disappeared on follow-up5,15,16. Our study, which employed a greater number of patients, failed to observe any significant difference in residual shunt among the groups at a median follow-up of 32 months.
DIFFERENCES AMONG THE DESIGNS
Cobra deformity was observed more often in the FSO due to its design, but it was neither statistically significant nor clinically relevant21. The lack of embolisation of an FSO could be due to the angulated pusher, which prevents a jump of the device upon release12. The tilt-rotatable pusher of the FSO could explain the relatively less frequent need for special deployment techniques compared to the other designs, but this did not reach statistical significance and it did not reduce the procedural time for the FSO4. The smaller discs in the FSO compared to the other designs were considered less thrombogenic and less arrhythmogenic, but the difference in the disc diameter of 1-2 mm was not clinically relevant4,15. The larger vascular access sheaths required for the CSO and FSO did not result in clinically significant vascular complications such as venous thrombosis, occlusions or bleeding6,13. Even though we failed to observe significant merits for the newer devices, a much larger comparative trial might show differences among the devices.
Limitations
In this prospective study with concurrent use of three different designs, the method of randomisation was not robust. The patients were serially allotted to the three groups in the order in which they were recruited into the study. The conventional method of random numbers would have resulted in an unequal number of patients in the three groups. However, the large number of patients in each group ensured comparability of the parameters. Another limitation was the evaluation of the safety and patient outcomes in the short term and medium term only. Long-term results beyond three years will be necessary to evaluate late complications such as erosions and delayed thrombosis on the larger devices. Since TEE and Holter studies were not routinely utilised on follow-up, small residual shunts, thrombus and atrial arrhythmias might have been missed.
Conclusions
This study, the first of its kind to the best of our knowledge to compare three similar nitinol wire-mesh atrial septal occluders prospectively and concurrently, demonstrated their equivalence in feasibility, safety and efficacy in transcatheter closure of atrial septal defects. Even though the defect diameter and device size were comparable among the groups, the delivery system was smaller with the ASO, but this did not translate into statistical differences in vascular access or procedural complications. Special techniques such as balloon assistance or pulmonary vein deployment were needed less often with the FSO, and cobra formation was noticed more often in the FSO compared to the other designs. However, this did not translate into variations in outcomes and adverse events. In spite of the modifications in the material and design of the newer devices, the complication rates were equally low with all the occluders. It is likely that the complications are related more to the anatomy of the defect and the conduct of the procedure rather than the occluder design. Further prospective, randomised, multicentre studies are required to compare the technical aspects, deficiencies and long-term outcomes of the three devices.
|
Impact on daily practice Different designs of nitinol occluder used for closure of secundum atrial septal defects are uniform in their efficacy, safety and provide similar outcomes in clinical practice. The AMPLATZER Septal Occluder, which is the prototype of such devices, often needs a smaller delivery system compared to the later designs, but this does not translate into a significant reduction in procedural and vascular access complications. As the transcatheter closure of atrial septal defects has evolved into a very safe intervention in the hands of an experienced operator, the modifications in the material, design and release mechanism offered by the newer designs do not lead to a further significant reduction in complications. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

