Abstract
Aims: Transcatheter closure of the secundum-type atrial septal defect (ASD) is widely practised. We report complications and efficacy of percutaneous ASD closure in adults using the Amplatzer® ASD occluder and the Cardioseal/Starflex® device during long-term follow-up.
Methods and results: Between 1996 and 2008 percutaneous ASD closure was performed in 133 patients (mean age 46.8±16.9 years; 36 men) by using the Amplatzer® device in 104 patients and the Cardioseal/Starflex® device in 29. During a mean follow-up of 3.4±2.8 years the occurrence of major complications was higher in patients with the Cardioseal/Starflex® compared to patients with the Amplatzer® devices (17.2 vs. 2.9%, log rank, P=0.005), due to a higher embolisation rate (13.8 vs. 1.0%, log rank, P=0.002). In univariable analysis, the implantation of a Cardioseal/Starflex® device (OR 6.0 (CI 1.4-25.2); P=0.01) and a larger device diameter (OR 1.1 (CI 1.0-1.2); P=0.04) were found to be predictors of the occurrence of major complications. Minor complications occurred in 10.5%, recurrent thrombo-embolism in 2.3% and residual shunting at six months was 13.9% without differences between devices. NYHA class improved from 1.8±0.6 before to 1.2±0.4 after closure (P<0.001) without differences between devices.
Conclusions: During long-term follow-up, percutaneous ASD closure in adults is safe and effective when using the Amplatzer® device. Larger Cardioseal/Starflex® devices are related to a higher embolisation rate. Randomised trials are needed.
Introduction
An atrial septal defect (ASD) accounts for about one third of all congenital heart diseases detected in adults.1 The secundum-type ASD is located in the region of the fossa ovalis and makes up 75% of all ASD’s.2 An ASD is characterised by predominant left-to-right shunting and right ventricular volume overload.2 Most patients with a sizable ASD remain asymptomatic during the first two decades of life.2 However, eventually, most develop symptoms due to cardiac failure, pulmonary hypertension, atrial arrhythmias and paradoxical embolism, the latter due to right-to-left shunting.1,3 To avoid the increase in the incidence of severe disability and death, surgical ASD closure used to be the only option that increased survival when shunting compromised life expectancy.4,5 Nowadays, transcatheter ASD closure is widely practised and has replaced to a large extent surgical closure in many centers.6
We report our experience in percutaneous closure of a secundum-type ASD in adults. We compared complications and efficacy of the Amplatzer® ASD occluder (AGA Medical Corporation, Golden Valley, MN, USA) and the Cardioseal/Starflex® device (NMT Medical, Inc., Boston, MA, USA) during long-term follow-up.
Methods
Patient selection
We included all patients (> 16 years of age) who underwent percutaneous ASD closure by means of an Amplatzer® ASD occluder or a Cardioseal/Starflex® device in the St. Antonius Hospital, Nieuwegein, The Netherlands between November 1996 and January 2008.
All secundum-type ASD’s were identified by transthoracic and transesophageal echocardiography (TTE and TEE) using colour Doppler techniques and/or contrast examination. Pre-, peri- and post-procedural data were collected, including demographic, echocardiographic, angiographic and procedural parameters. All patients gave informed consent.
ASD closure
As previously reported, ASD closure was performed according to standard techniques, under general anaesthesia and continuous TEE monitoring.7 The choice of the device type was made in accordance to the clinical preference of the interventional cardiologist.
Complications and efficacy
Follow-up information was obtained by periodical outpatient visits and a telephone interview. The most recently available medical records were reviewed. We compared data for the two ASD closure devices used.
Complications
Complications of transcatheter ASD closure, including periprocedural complications (occurring during hospitalisation) and complications during follow-up, were noticed. They were classified as major or minor according to the following scheme.
The primary endpoint was defined as the total of major complications related to percutaneous ASD closure. Major complications included haemorrhage requiring blood transfusion, occurrence of cardiac tamponade, need for procedure-related surgical intervention, massive fatal pulmonary emboli, occurrence of new thromboembolic event and death, related to the closing procedure.
Minor complications were defined as device malpositioning or embolisation with successful catheter repositioning, bleeding not requiring blood transfusion, occurrence of new-onset atrial arrhythmias (atrial flutter or fibrillation), transient atrioventricular block, device arm fractures, asymptomatic device thrombosis, need for re-catheterisation, transient air embolism, transient ST-segment elevation, femoral arteriovenous fistula formation, femoral haematoma, and other minor complications related to the closing procedure.
Efficacy
Some patients underwent ASD closure because of a paradoxical thromboembolism (stroke, TIA, or other thromboembolic event). Therefore, the recurrence of thromboembolic events was evaluated during follow-up. All neurological embolic events needed to be established by a neurological evaluation and, in case of stroke, confirmed by the appropriate cerebral imaging studies.
To evaluate symptoms related to the ASD, the New York Heart Association (NYHA) functional class was recorded before and after ASD closure by a telephone interview.
The presence of residual shunting after ASD closure was evaluated based on a contrast TTE or TEE study performed at least six months after ASD closure.
Statistical analysis
Descriptive statistics were used to report patients’ characteristics. Continuous variables were tested on normality and, if present, reported by mean±standard deviation (SD). Median and range were used when normal distribution was absent. Percentages were used to report categorical variables. Patients’ data were compared among groups with Chi-square or Fisher exact test for nominal variables and independent Student’s t-test for continuous variables. Paired samples t-test was used for within group comparison of continuous variables. The log rank test was used where applicable. Kaplan-Meier survival analysis was done on the primary endpoint and the occurrence of minor complications. Predictors of the primary endpoint and the occurrence of minor complications were assessed using a Cox proportional hazards model. Univariate logistic-regression analyses were used to estimate the unadjusted odds ratios (OR) and the corresponding 95% confidence intervals (CI). All tests were two sided and P<0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS software, version 12.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
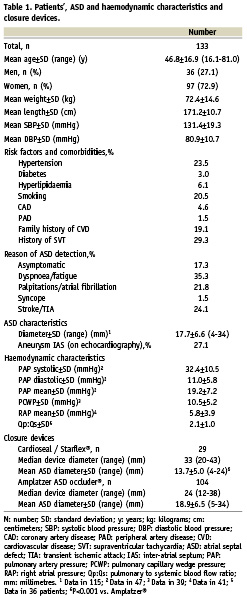
Between November 1996 and January 2008 percutaneous ASD closure was performed in 133 consecutive patients (mean age 46.8±16.9 years [range 16.1-81.0 years]; 36 men). Patient characteristics, prevalence of risk factors for cardiovascular disease, comorbidities and the reason of ASD detection are summarised in Table 1.
ASD and closure characteristics
On echocardiography before ASD closure, all shunts were bidirectional, but predominantly from left to right. An associated atrial septal aneurysm (ASA) was found in 27%.
Forty-seven patients (35%) underwent invasive determination of haemodynamic characteristics before closure. Mean Qp:Qs ratio was 2.1±1.0. Echocardiographic data and haemodynamic characteristics before ASD closure are summarised in Table 1. The mean ASD diameter on balloon sizing before ASD closure was 17.7±6.6 mm (range 4-34 mm). For ASD closure, we used the Amplatzer® ASD occluder in 78% and the Cardioseal/Starflex® device in 22%. In the beginning of the study period the Cardioseal/Starflex® device prevailed. Later on the Amplatzer® ASD Occluder was implanted in most cases. The mean ASD diameter was larger in patients that received an Amplatzer® device compared to patients that received a Cardioseal/Starflex® device: 18.9±6.5 vs. 13.7±5.0 mm (P<0.001). These data are summarised in Table 1.
Complications
Primary endpoint
The mean follow up time was 3.4±2.8 years (range 0-10.9 years) and differed between the two devices used for ASD closure (Table 2). The primary endpoint occurred in eight patients (6.0%). In five patients device embolisation occurred (two peri-procedurally and three during follow-up). In a 56 year-old man, an Amplatzer® 22 mm device embolised into the left ventricle during the procedure, requiring immediate surgery. The device was removed and the ASD was closed. Another embolisation occurred in a 34 year-old woman during implantation of a Cardioseal/Starflex® 43 mm device. The device embolised into the pulmonary artery and was successfully removed surgically combined with surgical ASD closure. A 49 year-old woman suffered embolisation into the pulmonary artery of a Cardioseal/Starflex® 40 mm device two weeks after the procedure. She required an operation to remove the device and close the ASD. In a 54 year-old man device embolisation into the pulmonary artery was diagnosed three months after implantation of a Cardioseal/Starflex® 40 mm device. The device was removed surgically and the ASD was closed with sutures. However, he needed re-operation because of rupture of a suture. Eventually, he received a Goretex patch, after which the ASD was closed. Finally, in a 61 year-old woman, migration of a Cardioseal/Starflex® 40 mm device in the interatrial septum was diagnosed five months after implantation. The device was surgically removed and a Goretex patch was used to close the ASD. Other major complications occurred in the following three patients: a 25 year-old woman suffered a haemopericardium immediately after having received a Cardioseal/Starflex® 28 mm device. The haemopericardium was probably due to perforation of the device or catheter and required pericardial drainage, which was successful. An 81 year-old woman required surgery for an inguinal pseudoaneurysm she developed one month after ASD closure with an Amplatzer® 24 mm device. The pseudoaneurysm was caused by inadvertent puncture and laceration of the femoral artery. Finally, a 41 year-old woman with a history of atrial arrhythmias suffered an episode of atrial fibrillation one week after implantation of an Amplatzer® 20 mm device. After electrical cardioversion in the referring hospital she suffered a stroke, probably due to an atrial thrombus, for which Coumadin was started.
The occurrence of the primary endpoint was significantly higher in patients that had received a Cardioseal/Starflex® device as compared to patients that had received an Amplatzer® device (17.2 vs. 2.9%; log rank, P=0.005) (Table 2). Kaplan-Meier event-free survival curves for the primary endpoint for patients with Amplatzer® and Cardioseal/Starflex® devices are plotted in Figure 1A. The higher occurrence of the primary endpoint in patients with Cardioseal/Starflex® devices is attributable to a higher embolisation rate of these devices compared to the Amplatzer® devices (13.8 vs. 1.0%, log rank, P=0.002) (Table 2). In patients who received Cardioseal/Starflex® devices, the initial ASD and device diameters were significantly higher in patients in whom the device embolised compared to the others, 18.8±3.8 vs. 12.9±4.7 mm for ASD diameter (P=0.03) and 40 (40-43) vs. 33 mm (20-40) for device diameter (P=0.004). In univariable analysis, the implantation of a Cardioseal/Starflex® device (OR 6.0 [CI 1.4-25.2]; P=0.01) and a larger device diameter (OR 1.1 [CI 1.0-1.2]; P=0.04) were found to be predictors of the primary endpoint.
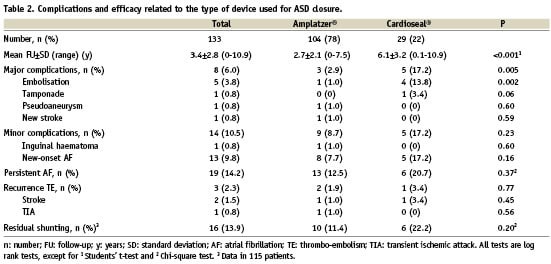
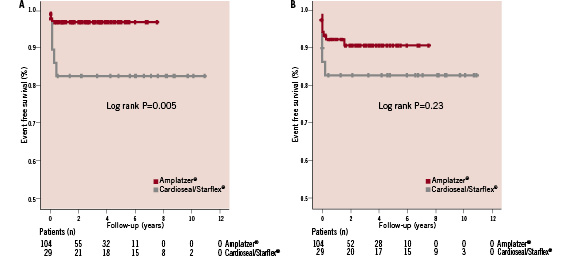
Figure 1. A. Kaplan Meier event free survival curves for the primary endpoint for the Amplatzer® and Cardioseal/Starflex® device. B. Kaplan Meier event free survival curves for the occurrence of minor complications for the Amplatzer® and Cardioseal/Starflex® device.
Minor complications
Minor complications related to the percutaneous ASD closure occurred in 14 patients (10.5%). Minor periprocedural complications (3.0%) consisted of three patients with new-onset atrial fibrillation (AF) successfully treated with medication or electrical cardioversion and one patient who suffered an inguinal hematoma, which was treated conservatively.
Minor complications during follow-up consisted of ten patients (7.5%) with new-onset AF. All were successfully treated with medication or electrical cardioversion. The occurrence of minor complications did not differ between the device types used (Table 2). Kaplan-Meier event-free survival curves for the occurrence of minor complications for both closure devices are plotted in Figure 1B. In univariable analysis, a higher age (OR 1.03 [CI 1.0-1.07]; P=0.04) was found to be a predictor of the occurrence of minor complications.
The occurrence of AF after ASD closure was high. However, 59% of these patients already had a history of AF. Thirteen patients (9.8%) suffered new-onset AF, whereas 19 patients (14.2%) with a history of AF suffered a new episode after ASD closure (Table 2). Device type or size and age did not influence the occurrence of new-onset AF.
In three patients, closure devices were removed during follow-up for different reasons, other than complications. One patient underwent a MAZE procedure for AF, during which the device was removed and the ASD was closed surgically. Another patient suffered from multiple ASD’s, as diagnosed during the closing procedure. Percutaneous closure of the largest ASD in this patient was successful and without complications. However, she had persistent symptoms due to the remaining ASD’s and eventually underwent surgical closure. The third patient, who had a history of paradoxical embolism, was diagnosed moderate residual shunting after an uncomplicated ASD closure. There was no dislocation. We advised Coumadin, however, the patient had the closure device surgically removed, followed by surgical ASD closure in another hospital.
Efficacy
Recurrent thromboembolic events
Recurrent thromboembolic events after ASD closure occurred in three patients (2.3%). The annual recurrent thromboembolic event rate was 0.7%. A 58 year-old woman with a history of multiple strokes, in whom a Cardioseal/Starflex® 28 mm device was implanted, suffered a recurrent TIA and stroke. Echocardiography revealed no residual shunting, however, there was a suspicion of left atrial thrombus, for which Coumadin was started. A 38 year-old woman with a history of stroke, in whom an Amplatzer® 35 mm device was implanted, suffered a recurrent stroke. Echocardiography revealed no residual shunting in this patient. Finally, a 36 year-old woman, with a history of TIA, in whom an Amplatzer® 16 mm device was implanted, suffered a recurrent TIA. No residual shunting was found on echocardiography. The recurrence of thromboembolic events did not differ between the two device types used (Table 2).
NYHA functional class
Analysis of NYHA functional class was obtained in 77 patients (58%). Mean NYHA class before ASD closure was 1.8±0.6 and decreased to 1.2±0.4 at latest follow-up after ASD closure (P<0.001). No differences between the two device types used were found.
Residual shunting
On periprocedural TEE, residual shunting after ASD closure was found in 23 patients (17.7%). At latest follow-up, residual shunting, as diagnosed with a contrast TTE (or TEE in some cases), was reduced to 13.9% based on analysis of 115 patients. There was no difference in the prevalence of residual shunting between the device types used (Table 2). Residual shunting was not a predictor of thromboembolism after ASD closure.
Discussion
Our results demonstrate that transcatheter closure of ASD’s of the secundum type in an adult patient population is safe and effective during long-term follow-up using an Amplatzer® device. However, using the Cardioseal® device is related to a high rate of major complications.
Surgical ASD closure
In the past, in patients with sizable ASD’s, surgical closure was the only option that increased survival.4,5,8 Historically, the operation in adults had an early mortality rate of 1.2-3.3% and moderate to severe complications were observed in 9-13% of patients.5,8,9 Recent comparative data between surgical and percutaneous ASD closure in children and adults described 0% mortality rates for both treatment modalities.10-12 However, a higher major complication rate of 10.5-25% was found in patients who underwent surgical closure as compared to 1-13.2% for percutaneous closure.10-12 Surgery was strongly related to the occurrence of total and major complications.10 The higher event rate was associated with age > 40 years.12 However, as for transcatheter closure techniques, surgical techniques have been improved as well. Mishra et al compared 470 patients who underwent transcatheter ASD closure with 170 patients taken for ASD closure through minimal invasive port access surgery and found both techniques to be equally safe and effective with a success rate of 97.1 and 99.4% and a major complication rate of 1.8 and 2.9%, respectively.13
Percutaneous ASD closure
The clinical feasibility of transcatheter ASD closure was first described by King and Mills in 1976.14,15 Since then the technique and devices have been continuously improved and have to a large extent replaced the surgical ASD closure. Percutaneous closure of a secundum-type ASD is nowadays widely practiced. There have been numerous reports that describe the safety and efficacy of transcatheter ASD closure.16-26
Complications
Major complications
In several studies major complication rates of 0-11.1% of transcatheter ASD closure have been reported, which is comparable with the rate of 6.0% we found.16-26 Importantly, we found a higher major complication rate in patients, who had received a Cardioseal/Starflex® device (17.2%) as compared to patients with an Amplatzer® device (2.9%). In univariable analysis, the presence of a Cardioseal/Starflex® device and a larger device diameter were predictors of the occurrence of major complications. These major complications were mainly attributable to device embolisation. We reported a total embolisation rate of 3.8%, which is comparable with embolisation rates of 0-7.4% reported in several studies.16-26 However, the occurrence of embolisation was significantly higher in patients, who had received a Cardioseal/Starflex® device (13.8%) as compared to patients with Amplatzer® devices (1.0%). In contrast, Butera et al found a non-significant difference in embolisation rate between these two devices.16 They reported embolisation in three (2.5%) of 121 patients who received the Cardioseal/Starflex® device as compared to one (0.7%) of 153 patients who received an Amplatzer® device.16 An explanation for the high embolisation rate using the Cardioseal/Starflex® devices in our study could be the larger diameter of the ASD and closure device in these patients. These diameters seem to be larger in our study compared to others who used the Cardioseal/Starflex® device.16,23 This is supported by the fact that initial ASD and device diameters were significantly larger in patients in whom the device dislocated or embolised compared to the others. However, the four Cardioseal/Starflex® devices that embolised or migrated were part of the first 30% of all transcatheter ASD closing procedures performed in our centre. We have to take into account a learning curve and the fact that closing techniques and some closure devices are continuously technically improved. During the last 70% of all procedures, only one device (Amplatzer®) embolised. Nowadays, we preferably use the Amplatzer® device in patients with large ASD’s and only consider Cardioseal/Starflex® devices in patients with small defects.
Minor complications
A minor complication rate of 0-37% has been reported for transcatheter ASD closure.16-26 These varying numbers are probably the result of differences in follow-up time, the definition of complications and the device types used.
In our study, minor complications occurred in 10.5%. Taking into account the follow-up period of three and a half years in our study, which is longer compared to other studies, our results are even more beneficial. We found no differences in the occurrence of minor complications between the two devices used. In univariable analysis, higher age was found to be a predictor of the occurrence of minor complications.
In our study new-onset AF was the most frequent complication (9.8%) after ASD closure. Spies et al found an annual incidence of new-onset AF of 4.1% in 240 patients after ASD closure during a median follow-up of 20 months27 compared to an annual incidence of 2.9% in our study. In our study, device type or size and age did not influence the occurrence of new-onset AF.
Efficacy
Recurrent thromboembolic events
The occurrence of thromboembolic events after ASD closure varies between 0% and 2.3%.16-26 In most studies, it remains unclear, whether these events are recurrent or not. We found recurrent thromboembolic events in 2.3% which is comparable with previous reports. However, the follow-up period of our study was longer compared to other reports. The chance of having a thromboembolic event increases with longer follow-up duration. Indeed, the annual recurrent event rate was only 0.7%. Additionally, recurrent thromboembolic events might not be related to ASD closure but to for instance systemic atherosclerosis. In support, in our study, the recurrent thromboembolic event in one patient could have been device related. This concerns the patient who was diagnosed possible left atrial or device thrombus. In the two other patients who suffered a recurrent thromboembolic event, no device thrombus or residual shunting could be found on echocardiography. These events were probably not device-related.
NYHA functional class
An improvement in symptoms and exercise tolerance has been reported in patients undergoing percutaneous ASD closure.23,28 We also found an improvement in NYHA functional class after ASD closure. This finding is compatible with the improvement in exercise capacity and cardiac function in adult patients after ASD closure reported by Giardini et al.29
Residual shunting
Residual shunting during follow-up after ASD closure has been reported in 0-50%.16-26 These differences can be explained from the differences in follow-up time, the definition of residual shunting and the use of different imaging modalities to diagnose residual shunting. We found residual shunting, as diagnosed by a contrast TTE (and in a few cases TEE) and including very small shunts, in 13.9% of patients after ASD closure at latest follow-up. No differences were found between the two devices used.
Limitations
The first limitation of our study is the single centre character. The second limitation is that data on residual shunting en NYHA functional class were not available in all patients. The third limitation is that the comparison of the Amplatzer® and the Cardioseal/Starflex® device could be affected by the continuous technical improvement of the closing technique and the devices.
Conclusion
Percutaneous closure of a secundum-type ASD in adults is safe and effective, especially when using the Amplatzer® device. The larger Cardioseal/Starflex® devices are possibly related to a higher rate of embolisation. Prospective randomised trials that compare different types of devices are needed.

