Abstract
Aims: The aim of this study was to assess the midterm results of percutaneous closure of very large atrial septal defects (ASD) in children with transthoracic echocardiography (TTE) and multislice computed tomography (MSCT).
Methods and results: Among 142 children who underwent percutaneous ASD closure with the AMPLATZER® Septal Occluder (ASO) (AGA Medical Corporation, Plymouth, MN, USA) during an eight year period, 51 patients with very large defects, were evaluated by TTE and MSCT after a period of at least two years following ASD closure. Median age at ASD closure was six years (range 4-10), with mean ASD size 20.9±2.9 mm. Median device size was 20 mm (range 15-26) and median device:septal length ratio 0.95 (range 0.8-1). Early complications included one transient complete atrioventricular block and one device embolisation. At a median follow-up of 55 months (range 25-92) all patients were clinically asymptomatic and had a normal ECG. TTE did not demonstrate device protrusion across the lumen of either the systemic or pulmonary veins. The mean device:septal length ratio had decreased from 0.96±0.05 to 0.8±0.02 (p<0.001). There was good correlation between the measure of atrial septum length by TTE and MSCT (r: 0.79, p<0.001). MSCT identified moderate dynamic device protrusion into the lumen of systemic or pulmonary veins in five patients and partial device malpositioning in two patients.
Conclusions: Occlusion of very large ASD in children can be performed with low complications rate. MSCT provides detailed information regarding the location of the device with respect to surrounding anatomic structures and reveals anomalies not evident by TTE.
Introduction
Atrial septal defects (ASD) are a common congenital anomaly that may induce right ventricular volume overload. Ostium secundum ASD are generally closed percutaneously in most centres with good long-term results1. When not closed during childhood, moderate to large ASD can lead to serious complications in adulthood, such as arrhythmias, pulmonary hypertension and congestive heart failure2.
Rarely, percutaneous closure of large ASD may lead to device embolisation, erosion of the atrial or aortic wall3-5, atrioventricular block6 and device protrusion into the lumen of systemic and pulmonary veins or through the atrioventricular valves7. After closure of very large ASD, transthoracic echocardiography (TTE) may be limited by poor acoustic windows that lead to an incomplete assessment of the device. Because of its excellent spatial resolution, multislice cardiac computed tomography (MSCT) has been used to evaluate cardiac abnormalities while assessing the results of ASD closure by the AMPLATZER Septal Occluder (ASO)8.
Although a large device may appear bulky when implanted in a child, growth increases the length of the interatrial septum as well as the distance between the device and the adjacent cardiac structures9. In order to verify this hypothesis and to rule out the possibility of late obstruction of systemic or pulmonary veins or device protrusion across the atrioventricular valves, we prospectively evaluated 51 consecutive children with TTE and MSCT who underwent percutaneous closure of large ASD in a single institution.
Patients and methods
Between January 2000 and December 2007, 142 children underwent successful transcatheter ASD closure at the Necker Enfants Malades Hospital, Paris, France with the ASO. Only patients with isolated secundum ASD larger than 15 mm/m2 with superior, inferior and posterior rims of at least 4 mm and a QP/QS ratio >2 were included in the study. Exclusion criteria were: ASD associated with other forms of congenital heart disease, ASD with right to left shunt, those defects close to the coronary sinus ostium or ASD too large compared to the length of the interatrial septum (see below). Fifty-one patients met the above inclusion criteria.
In order to evaluate the midterm results these children were enrolled for evaluation by TTE and MSCT after a period of at least two years.
The institutional ethics committee approved the study. Consent for MSCT was not obtained in three of the 51 cases.
Patient characteristics
Reasons for ASD closure were isolated right ventricular overload in 43 patients and right ventricular overload associated with frequent respiratory infections in six, and to reduced tolerance to exercise in two. Median age, weight and body surface area of patients at ASD closure were six years (range 4-10), 21 kg (range 12-46) and 0.8 m2 (range 0.5-1.4), respectively.
Transthoracic echocardiography (TTE)
All children underwent two-dimensional TTE, Doppler investigation and colour Doppler mapping by a VIVID 7 echocardiography machine (General Electrics Medical System, Milwaukee, WI, USA) prior to attempted device closure. The maximal length of the interatrial septum was measured in the subxyphoid and four chambers views. The length of the interatrial septum was the mean of three consecutive measurements in the two different views. The position of the defect was evaluated, its diameter measured in at least three different views (subxyphoid, four chambers and short axis) and the size of superior, posterior and inferior rims assessed. In order to evaluate the feasibility of percutaneous closure, we added 12 to 14 mm to the measured defect diameter (in ASO sized less than 11 mm, the left atrial disc diameter was 12 mm longer than the waist diameter; in ASO sized between 11 and 32 mm included, the left atrial disc diameter was 14 mm longer than the waist diameter). When calculating the device:septal ratio we considered the left atrial disc diameter.
ASD closure
The technique of ASD closure has been previously described3. All procedures were performed under general anaesthesia and guided by transesophageal echographic (TEE) with closure in all cases using the ASO device.
In order to choose the appropriate device size we measured the distance between the solid rims of the defect, as previously described10 or inflated a compliant balloon over the guidewire through the atrial septum, until suppression of interatrial shunt occurred (stop-flow technique). TEE was repeated after device delivery to evaluate any residual shunt, and to highlight any possible impingement of the device on relevant cardiac structures and to rule out the presence of pericardial effusion.
Follow-up
Median age, weight and body surface area of patients at follow-up were eight years (range 6-12), 23 kg (range 15-52) and 0.9 m2 (range 0.6-1.5). Fifty-one patients had TTE and 48 MSCT, both performed on the same day. TTE evaluation was aimed at measuring the device:septal length ratio in the subxyphoid and four chambers views and at excluding the presence of obstruction to systemic and pulmonary venous flow (defined as the presence of turbulent flow on colour Doppler or peak velocity higher than 1m/sec on pulsed Doppler), or protrusion of the device across the annulus of atrioventricular valves. TTE was performed the day before ASD closure and at the follow-up, and the results obtained with TTE and MSCT were compared.
MSCT
All examinations were performed with a 64-slice scanner (General Electrics Medical System, LightSpeed VCT, Milwaukee, WI, USA). The standard acquisition protocol included 370 ms rotation time, 0.625 mm of slice thickness, pitch 0.16, voltage 80 kV, current varied during acquisition and adapted to the weight of the child. A bolus of 1.5 ml/kg of iodine contrast medium (lohexol 300 mg/ml of iodine; Schering SA, Berlin, Germany) was injected at a rate of 0.5 to 2.5 ml/sec with a power injector (Medrad En Vision Computed Tomography, Pittsburgh, PA, USA), followed by a chaser bolus of 5 to 10 ml of saline.
Patients older than six years of age were instructed to inspire and hold their breath during data acquisition. Younger patients were allowed to breath freely.
We performed acquisition with an ECG-gating technique, at 75% R-R interval, and completed retrospective reconstructions at every 10% R-R interval. Data sets were analysed by two experienced independent investigators.
The device and atrial septum were examined in multi-planar views (axial, coronal, sagittal and oblique). Post-processing tools, such as multi-planar reconstruction and maximum-intensity thin-slab projection were used in all cases in order to define the anatomy of the atrial septum and obtain projections corresponding to the standard echocardiographic views8,10,11. The maximal length of the interatrial septum was measured in the four chamber view and in the oblique view passing through the superior and inferior caval veins (Figure 1). The length of the interatrial septum was calculated as the mean of three consecutive measurements.
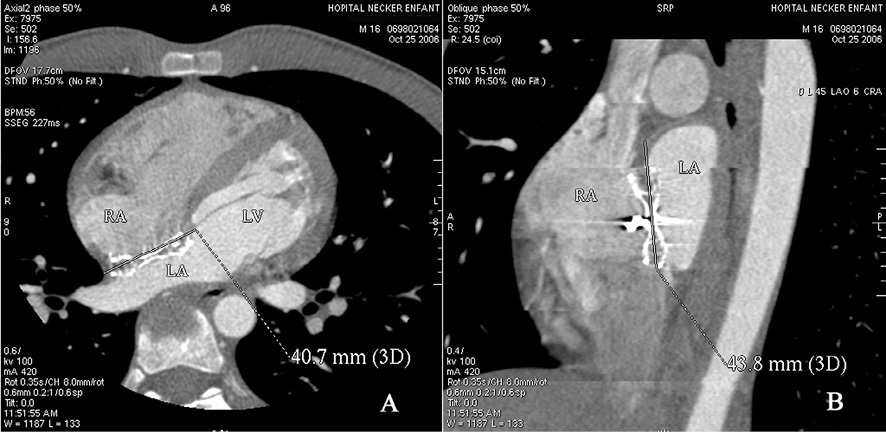
Figure 1. Measurement of interatrial septum length in 4 chambers and oblique view.
All acquisitions were studied every 10% R-R interval and dynamically during the cardiac cycle in order to observe the movements of the device and to detect any friction with adjacent structures. If device protrusion in the lumen of systemic and pulmonary veins was present, it was evaluated at end-systole and end-diastole and expressed as a percentage.
The effective dose of MSCT was calculated by the method proposed by the European Working Group for Guidelines on Quality Criteria in CT12. The effective dose, derived from the product of the dose-length product (DLP) and conversion coefficients for the chest, taking into account patient age, as proposed by Shrimpton and Wall13, was 4.7±0.6 mSv.
Statistical analysis
Descriptive statistics for the total population were obtained. Age and weight were quoted as median with ranges; continuous variables were presented as the mean plus or minus standard deviation. Comparison for individual parameters was performed using the two-tailed paired t-test (if normally distributed). Linear regression analysis was performed using the correlation coefficient, r. A two-sided p-value of 0.05 or less was considered to indicate statistical significance. Statistical analyses were conducted with R software, version 2.00 (Insight Corporation, Boonton, NJ, USA).
Results
Early results
On preprocedural TTE, mean ASD size was 20.9±2.9 mm; mean septal length at time of ASD closure was 34.4±3.3 mm. Median device size was 20 mm (range 15-26) and median device:septal length ratio 0.95 (range 0.8-1).
ASD occlusion was achieved in all patients. Immediate occlusion rate was 87%. Incomplete occlusion was due to trivial to mild intra-prosthetic shunt. Residual paraprosthetic shunt was not seen. TTE did not show obstruction of adjacent vascular structures. In particular, device protrusion through the lumen of systemic veins and right superior pulmonary vein was not noticed. The device embraced the retroaortic rim or the aortic root when the retroaortic rim was absent. In one patient who experienced transient atrioventricular block, there was mild protrusion of the device into the mitral annulus, accompanied by trivial mitral incompetence.
There were two early complications: one transient complete atrioventricular block and one device embolisation to the pulmonary artery, which occurred the day following closure and was retrieved percutaneously, followed by insertion of a larger device. Transient migraine occurred in four patients who had a normal neurological exploration.
Midterm results
After a median follow-up of 55 months (range 25-92) all patients were asymptomatic and had a normal ECG. TTE did not demonstrate any device protrusion into the lumen of systemic and/or pulmonary veins. Device:septal length ratio had decreased from 0.96±0.05 to 0.81±0.02 (p<0.001).
MSCT showed that the device:septal length ratio was 0.84±0.07. There was good correlation between the measurement of atrial septal length by TTE and MSCT (r: 0.79, p<0.001).
MSCT revealed five patients had mild to moderate device protrusion through the lumen of superior vena cava (one patient) (Figure 2), inferior vena cava (three patients), right superior pulmonary vein (two patients) (Figure 3). There were no patients with device compression of the coronary sinus or residual shunt.
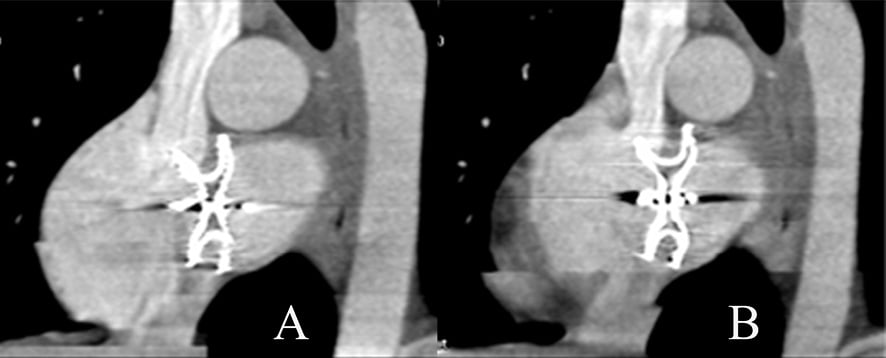
Figure 2. Device (ASO 24 mm) protrusion into the lumen of the superior vena cava in oblique view during systole (A) and diastole (B).
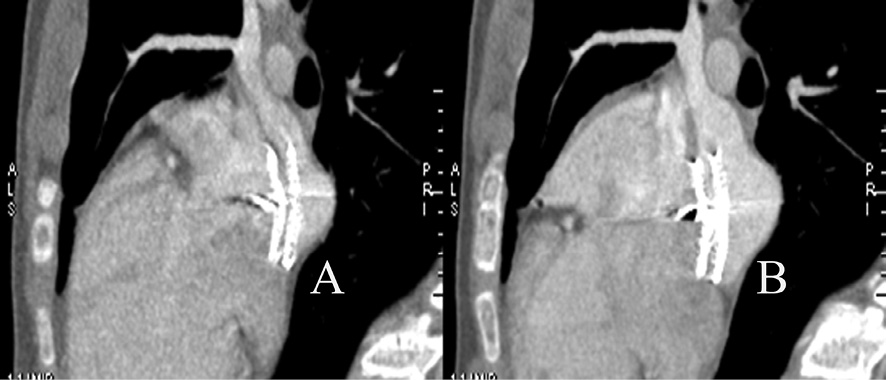
Figure 3. Device (ASO 20 mm) protrusion into the lumen of the right superior pulmonary vein in the oblique view during systole (A) and diastole (B).
The degree of device protrusion changed during the cardiac cycle; it was more prominent in diastole when assessing the relationship to the superior vena cava and the right superior pulmonary vein, and similarly in systole when the inferior vena cava was evaluated (Table 1). Indeed, the device movement during cardiac cycle followed the interatrial septum that was displaced anteriorly and inferiorly in systole, and posteriorly and superiorly in diastole.
In seven patients with absent aortic rim the device embraced the aortic root during the cardiac cycle (Figure 4A). In two patients, in whom TTE and TEE were reported as normal, MSCT revealed that the device, although occluding the septal defect, partially prolapsed into the right atrium (Figure 4B and Figure 4C).
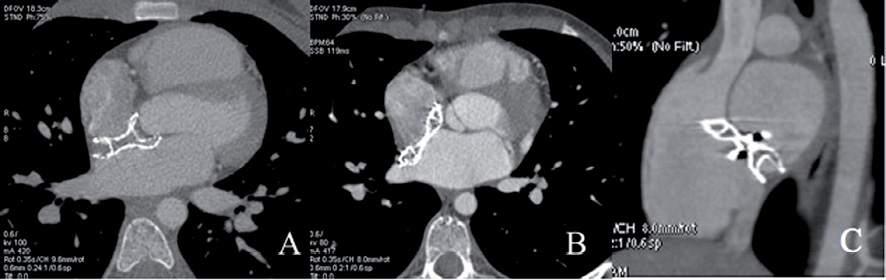
Figure 4. (A) Axial view showing the device (ASO 18 mm) embracing the aorta. The devices (ASO 24 and ASO 22 mm, respectively) prolapsed into the right atrium in two patients as shown in the axial view (B) and in the oblique view passing through the superior and inferior caval veins (C).
In one patient who had mild protrusion of the device through the mitral annulus by TTE immediately after the procedure, MSCT confirmed the device protrusion, but did not show any contact between the device and mitral leaflets two years after the procedure (Figure 5).

Figure 5. Relation between the device (ASO 26 mm) and the mitral valve (two different views) in a patient who experienced transient complete atrioventricular block.
Discussion
This study demonstrates that occlusion of very large ASD in children can be achieved with low immediate complications rates and that body growth reduces the device:septal length ratio, possibly diminishing the risk of mechanical trauma to adjacent cardiac structures.
Moreover, we highlight that, unlike TTE, MSCT reveals subclinical anomalies such as device malpositioning or device protrusion across the ostia of systemic or pulmonary veins.
The ASO device is largely used to occlude ASD in both paediatric and adult patients; long-term results are good and complication rates relatively low1-7,14. Although the closure of large ASD by ASO is effective and safe at short to intermediate term, the bulky profile of this device raises some concern regarding its long-term safety especially in children15. Well-known complications observed after ASO implantation include cardiac erosion, device malpositioning and embolisation3,16. Large device size seems to be accompanied by a higher complication rate7. However, in our series we did not observe any cardiac erosion and had only one early embolisation.
Arrhythmic problems often complicate the percutaneous closure of ASD3,6. Supraventricular arrhythmias are generally transient in young patients and were reported early after ASO implantation3; however the occurrence of atrioventricular block has also been reported6. A larger shunt and device size over 19 mm were the only determinant factors for transient atrioventricular block in this study6. In our series, one periprocedural atrioventricular block was likely due to the low position of the device, which protruded into the mitral annulus provoking trivial valvular insufficiency and a possible impingement on the penetrating bundle.
It has been previously shown that large ASO profile decreases during follow-up, resulting in a much lower device profile, especially when very large ASO are implanted15. Moreover, the distance between the device and local structures after ASD closure increases with body growth9. According to these results, our study demonstrated that body growth reduces the device:septal length ratio; thus, we can suppose that cardiac trauma due to direct contact between the device and adjacent structures and the dynamic vascular obstruction may become less relevant with time. This speculation is supported by the registry published by Amin et al in 2004, which reported that cardiac erosion occurred in patients less than 18-years-old within two days from ASD closure14.
Most cardiac erosions occurred early after implantation in adults also14. However, to the best of our knowledge the latest erosion reported in the literature occurred six years after device placement17. This 46-year-old patient underwent a 14 mm ASO implantation. Interestingly, the occluder device did not appear properly aligned with the superior limbus of septum secundum in retrospective analysis17.
An analysis of multicentric registries aimed to highlight the relationship between age at ASO implantation and timing of cardiac erosion, and between erosion and imperfect device position should be interesting in this setting.
After closure of very large ASD, TTE might be limited by poor acoustic windows that lead to incomplete device assessment. In fact, it is often difficult to assess the relationship of the device to the pulmonary and systemic veins or estimate the degree of device protrusion by conventional TTE. A previous study has shown that MSCT is useful in the assessment of symptomatic patients after AMPLATZER device implantation18. ECG-gated MSCT provided detailed information regarding the location of the device with respect to the aorta, atrial free wall and systemic veins. Compared to TTE it offered superior spatial resolution, allowing a unique assessment of the device in relation to surrounding anatomic structures and their dynamic displacement during the cardiac cycle8. The metallic component of the ASO, which might be a problem for magnetic resonance imaging and echocardiography, does not impair the diagnostic quality of MSCT. Because post-processing tools can reformat retrospectively any plane for any cardiac phase from its own acquisition data, MSCT may detect residual defects, shunts and protrusion not seen by TTE and measures the right ventricle volumes after ASD closure8,11. However, this technique is not suitable for routine or repeated examinations after ASD closure because of the radiation exposure, especially in young patients.
Cardiac magnetic resonance imaging (MRI) has been successfully used to evaluate the position of large ASO with regard to adjacent cardiac valves and veins19. Moreover, cardiac MRI is the gold standard for the volumetric analysis of the right ventricle after ASD closure20.
The main weaknesses of this technique are the need for general anaesthesia or prolonged sedation in case of uncooperative children, the presence of more consistent artefacts especially in free-breathing young children and the need for multiple oblique 2D steady-state free precession sequences19.
In the present study we sought to investigate the midterm results in a selected population of children in whom a large ASO device was implanted, and were therefore theoretically at risk of immediate or late complications. In our series, we found partial device protrusion and/or dynamic obstruction of systemic and pulmonary veins, not evident by TTE evaluation in seven out of 48 patients. Even if all our patients were completely asymptomatic, device malposition and venous obstruction late after ASD occlusion by ASO have been previously described and they raise concern about possible long-term complications21. Moreover, because MSCT and MRI are not routinely performed after implantation of large devices, the incidence of these anomalies might be underestimated in published series.
We can assume that our patients were asymptomatic because of the dynamic nature of the obstruction to venous return, which was never higher than 50%.
The main clinical implication of this study is that an increment in body growth is accompanied by a decreased device:septal length ratio, and that this favourable spatial rearrangement probably minimises the risk of late cardiac complications at least for small and moderate devices.
The main drawback of this study is the radiation dose exposure, which is a major concern in children, even if recent technology advances allow ECG-gated examinations with very low radiation exposure22. However, our cohort of patients will not undergo further or repeated cardiac MSCT. In clinical practise, cardiac MRI should always be preferred as a second level examination in growing patients during the follow-up, reserving cardiac MSCT for selected cases such as in symptomatic patients or when a device malposition is suspected by cardiac RMI or TTE.
In conclusion, even if device malposition and moderate dynamic obstruction to pulmonary and systemic venous return is well tolerated at midterm follow-up, longer-term and close clinical follow-up is necessary to rule out late complications after percutaneous closure of very large ASD in children.
Conflict of interest statement
The authors have no conflicts of interest to declare.