Abstract
Aims: To evaluate the safety and efficacy of the new Cera septal occluder (CSO) for atrial septal defect (ASD) closure as compared to the AMPLATZER Septal Occluder (ASO).
Methods and results: A total of 405 ASD patients receiving CSO or ASO were studied. The ASDs were classified into simple defects (isolated defects <26 mm) or complex defects (isolated defects ≥26 mm, large defects with a deficient rim, double or multi-fenestrated defects). Clinical and echocardiographic findings were collected before discharge, at one month, and every six months after implantation. Two hundred and five (133 females, aged 30±13 years) and 200 (135 females, aged 28±14 years) patients received CSO and ASO implants, respectively. The CSO group had similar ASD and device sizes, prevalence of complex lesions, procedural times and success rates as compared to the ASO group. Echocardiographic follow-up at one and six months showed similar residual shunts between devices. Both groups had similar rates for device embolisation and atrial arrhythmia. The average equipment cost per patient was lower in the CSO group than in the ASO group (US$3,500 vs. US$5,600, p<0.001).
Conclusions: Transcatheter ASD closure with CSO is safe and effective. It appears to be an attractive alternative to ASO because of its relatively low cost.
Introduction
Atrial septal defect (ASD) is the most common form of congenital heart disease and accounts for 7 to 10% of all forms of congenital cardiac defects in adults1. It should be closed in indicated patients to prevent pulmonary hypertension, arrhythmias, paradoxical embolism and heart failure due to right heart volume overload1,2. Previous studies have shown decreased right heart size, pulmonary pressure and arrhythmias soon after percutaneous ASD closure3,4.
Most ASDs can now be safely and effectively corrected through a transcatheter approach4-7. A number of devices have been developed for this purpose7-11. The AMPLATZER Septal Occluder (ASO) (St. Jude Medical Inc., St. Paul, MN, USA) has become the leading device worldwide for closing ASDs in the last two decades owing to its novel design, ease of use and proven sustained efficacy. The ASO is a self-expandable and self-centring double-disc device consisting of a nitinol wire mesh. The device is fully recapturable and repositionable if necessary.
However, the cost of the ASO remains a problem that restricts its application in many parts of the world. The Cera septal occluder (CSO) (Lifetech Scientific Corporation, Shenzhen, China) is a new, less expensive, self-expandable, double-disc device. Its nitinol frame is covered with a bioceramic titanium nitride (TiN) coating12,13. In the present study, we evaluated the safety and efficacy of closing secundum ASD using the CSO as compared to the ASO in 405 ASD patients.
Methods
STUDY POPULATION
Four hundred and five consecutive patients who received percutaneous ASD closure with either the CSO or the ASO between July 2004 and May 2012 were studied. All patients received transthoracic (TTE) and transoesophageal echocardiography (TEE) prior to intervention. All the participants had a diagnosis of secundum ASD with right heart dilation, and the maximal diameter (unstretched diameter) of the defect was <38 mm as measured by TTE. The ASDs were further classified into simple (isolated defects <26 mm) or complex defects (isolated defects ≥26 mm, large ASDs with a deficient rim, double or multi-fenestrated defects). Deficient rim was defined as having at least one adjacent atrial septal length <4 mm at anterior, inferior or posterior aspects of the ASD. Patients found to have a large defect (>38 mm), small shunt (Qp/Qs <1.5:1) or pulmonary vascular resistance of >8 Wood units under 100% oxygen assessed during cardiac catheterisation were excluded from the study. Informed consent was obtained from all participants or their relatives and the study was approved by the institutional review board. Aspirin and antibiotics were given 12 hours and one hour before the procedure, respectively.
CERA SEPTAL OCCLUDER AND AMPLATZER SEPTAL OCCLUDER
The Cera septal occluder (CSO) (Lifetech Scientific Corporation) is a new self-expandable device with two discs, the left disc being larger in diameter than the right disc. It is made of a nitinol wire mesh similar to the AMPLATZER Septal Occluder. The discs are connected by a short (4 mm) connecting waist (Figure 1). The Cera delivery system comprises a delivery sheath, dilator, loader, haemostasis valve, and a delivery cable with plastic vice.

Figure 1. Cera septal occluder device.
In contrast to the ASO, the two discs of the CSO consist of less metal, and the bioceramic TiN coating on the disc surface potentially minimises the risk of thrombus formation, causes less nickel ion elution, and facilitates endothelial tissue growth. The CSO device is available in nineteen sizes with the diameter of the connecting waist (size of the occluder) ranging from 6 mm to 42 mm, while the AMPLATZER device is available in twenty-six sizes (to accommodate various defect sizes) ranging from 4 mm to 38 mm. The sizes of the Cera delivery sheaths vary from 7 to 14 Fr whereas the AMPLATZER delivery sheaths range from 6 to 12 Fr.
IMPLANTATION PROCEDURE AND FOLLOW-UP
All patients received invasive haemodynamic evaluation before closure. The pulmonary arterial pressures were obtained with standard fluid-filled catheters. With oxygen uptake at rest, the Qp/Qs ratio is calculated by oximetry using Fick’s principle. Patients were given 75-100 units/kg heparin intravenously to achieve ACT >250 s during the procedure. Percutaneous closure was performed with continuous TTE or TEE guidance (Figure 2). The balloon sizing method was used to evaluate the size of occluder needed. The sizing balloon was tracked to the defect through the stiff wire positioned at the left upper pulmonary vein under echocardiographic guidance. The diameter of the balloon was increased slowly until the echocardiographic 2D colour Doppler flow disappeared at the defect. The stretched balloon diameter (SBD) was measured under echocardiography when the balloon occluded the defect completely. The stop-flow SBD diameter was used as reference for selection of device size in patients. In general, an occluder 1-2 mm larger than the SBD was chosen, while oversizing of the device was needed in patients with a flimsy septum or larger defects with relatively deficient rims14-16. The occluder was placed using the standard technique. The position and stability of the device were checked by the Minnesota manoeuvre16. The procedure was repeated in patients with double ASDs. Presence of residual shunt was checked with echocardiography. Finally, the device was released by disconnecting the delivery cable from the occluder. Acute procedural success was defined as stable and proper placement of the occluder to the defect. Residual shunt was represented by the presence of colour Doppler echocardiographic flow along the edge of the implanted occluder. The degree of residual shunt was classified according to the protocol reported by Boutin et al17, i.e., as trivial if the width of the colour jet as it exited the septum was ≤1 mm, small if it was ≤1-2 mm, moderate if it was ≤2-4 mm, and large if it was ≥4 mm. Successful closure was defined as any case with complete closure or a residual shunt jet width of ≤2 mm. The presence of trivial shunt through the device immediately after deployment was considered not clinically important.
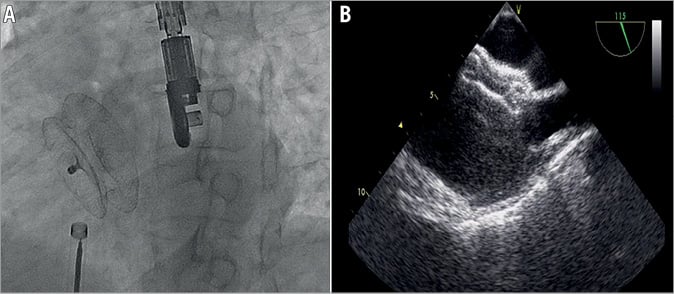
Figure 2. Transcatheter closure of an atrial septal defect with the Cera septal occluder. Angiographic (A) and transoesophageal echocardiographic (B) views.
A chest radiograph and TTE were performed at day one post implantation to exclude device embolisation and pericardial effusion, respectively. Clinical follow-up and TTE and/or TEE were arranged at one, six and 12 months after device implantation to assess the clinical outcomes and monitor treatment results. All patients were prescribed clopidogrel for one month and aspirin for six months after the procedure.
COST ASSESSMENT
We measured the costs of the procedure according to the social insurance institution records of the Turkish Ministry of Health from the beginning to the end of hospitalisation.
OCCURRENCE OF EVENTS
The safety endpoints were defined as occurrence of events related to aspirin or heparin (e.g., intracranial or gastrointestinal bleeding, other major bleeding requiring transfusion), or procedure (e.g., catheter-related thrombus formation, air embolism, serious pericardial effusion, acute device embolisation, procedure-related transient ischaemic attack or stroke). Endpoints including the presence of thrombus at the device, residual shunt, progressive valvular regurgitation, aortic wall erosion, or delayed device embolisation were assessed with TTE and/or TEE during the follow-up period. Clinical endpoints collected during follow-ups included new onset arrhythmias and cardiovascular death.
STATISTICS
All analyses were performed using SPSS version 15.0 for Windows (SPSS, Chicago, IL, USA). Categorical variables were expressed as absolute numbers or percentage and were compared using the chi-square test, whereas the continuous variables were summarised by mean±standard deviations and comparisons were made using the Mann-Whitney U test or unpaired Student’s t-test as appropriate. A p-value <0.05 was considered to be significant.
Results
CLINICAL CHARACTERISTICS
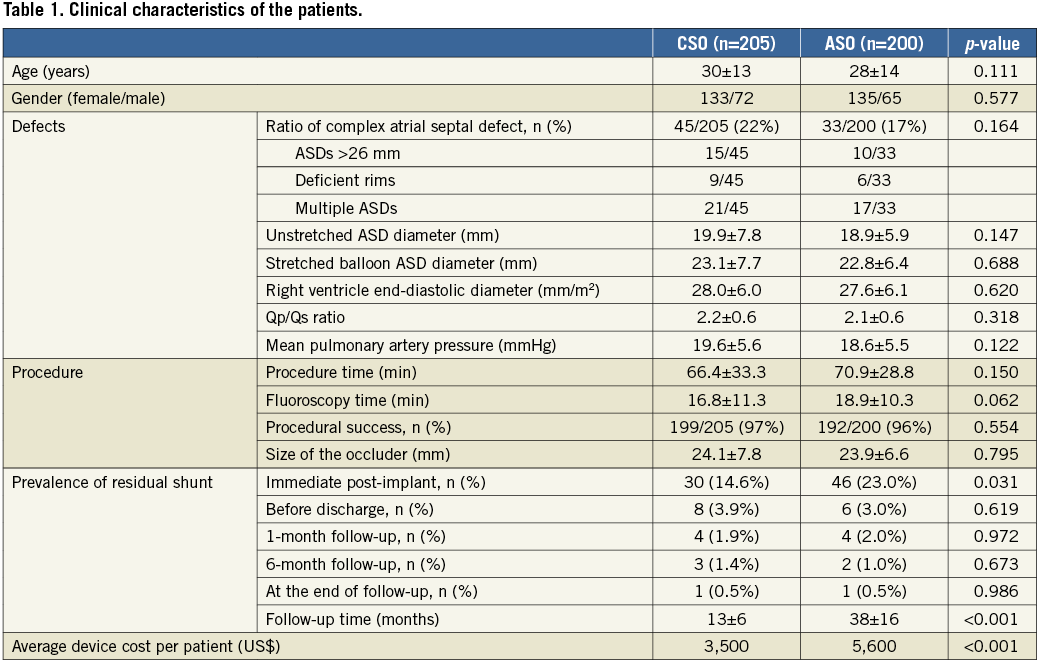
Demographic, echocardiographic and procedural data of patients are listed in Table 1. During the study period, 205 (133 females, mean age 30±13 years) and 200 (135 females, mean age 28±14 years) patients received CSO and ASO, respectively. The CSO group had a similar prevalence of complex lesions (22% vs. 17%, p=0.164) and a similar acute procedural success rate (97% vs. 96%, p=0.554) as compared to the ASO group. Fifteen patients had double ASDs: 12 of them received CSO and the remaining three patients received ASO. Also, the two patient groups did not differ in ASD (both unstretched/balloon-stretched diameters) and occluder sizes. The procedural times were similar in both groups, but the CSO group had a shorter fluoroscopy time (16.8±11.3 vs. 18.9±10.3 minutes, p=0.062). The average equipment cost per patient was lower in the CSO group than in the ASO group (US$3,500 vs. US$5,600, p<0.001).
ADVERSE EVENTS
No major bleeding event was observed during the procedure. There was no serious procedure-related complication except for the occurrence of acute device embolisation in four patients (one with CSO and three with ASO). We were able to retrieve the CSO from the pulmonary artery and the patient’s ASD was subsequently occluded by a larger CSO. The other three patients receiving ASO required surgical removal of the devices and repair of the ASDs.
All the devices were in a good position during TTE follow-ups. No device-related thrombus formation, device erosion into the aortic wall, progressive valvular regurgitation or delayed occluder embolisation was observed. No cardiovascular death occurred during follow-up. New-onset cardiac arrhythmia was detected in seven patients within one month after ASD closure (three supraventricular tachycardias [SVT] and one atrial fibrillation in the CSO group, and two SVTs in the ASO group). Rate-controlling agents were administered to patients developing SVT or atrial fibrillation, resulting in symptomatic relief. In our study, we also recognised three first-degree and one second-degree atrioventricular block in the CSO group, and two first-degree and one complete atrioventricular block in the ASO group. The patient with complete heart block was treated with a course of high-dose dexamethasone and the heart block disappeared after one week of therapy.
Discussion
This is the first multicentre study testing the efficacy and safety of the new Cera septal occluder for closing secundum ASD. We showed that ASD closure with the CSO is feasible and safe. The immediate, short-term and midterm closure rates and safety properties of the CSO are comparable to those of the ASO.
Early reports of transcatheter closure of ASD were published in 1976 by King and Mills18. Subsequently, over the last three decades, various devices have been used for percutaneous ASD closure. The initial and intermediate follow-up results of transcatheter closure of ASD are satisfactory with most devices18-20. Among them, the ASO has become the most widely used device worldwide with excellent safety and sustained efficacy results in both paediatric and adult patients8,21-23. The CSO is another device which has been developed to close secundum ASDs of varying sizes.
In the present study, we have reported the largest multicentre experience with CSO. Similar to the ASO, CSO is a self-expandable, nitinol-based device with two symmetric discs. The CSO differs from the ASO in that the nitinol frame is coated with a bioceramic titanium nitride (TiN) layer. The inner parts of the CSO’s nitinol frame, which contain the two discs and the central waist, are covered with an ePTFE membrane. One of the major limitations of the CSO is the requirement for larger delivery sheaths, sometimes up to 14 Fr, in closing large defects. This may increase the risk of vascular complications, especially in paediatric patients. However, this complication was not observed in our series. On the other hand, the CSO has several potential advantages as compared to the ASO.
First of all, the average equipment cost per patient was lower in the CSO group. Secondly, the CSO has a bioceramic TiN coating which can work as a mechanical barrier to block the rapid release of nickel in nitinol into the human body and has a surface with better biocompatibility and corrosion resistance24,25. Therefore, the CSO device causes less nickel toxicity and achieves earlier endothelialisation than a bare nitinol frame. Thirdly, considering the range of CSO sizes up to 42 mm, the CSO may be the preferred option in patients having extremely large ASDs13.
The implantation technique of the CSO is also similar to that of the ASO, and no additional skill is needed to deploy this new device. Furthermore, we demonstrated that it was possible to implant the CSO in patients with large or double defects. In 21 patients with large defects and relatively deficient rims, in whom standard deployment failed, we attempted the left upper pulmonary vein method26 with success and without serious complications. We also deployed the CSO in 12 patients with double ASDs, and all the smaller CSOs were firmly sandwiched between the two discs of the larger CSOs.
In experienced hands, complications were rare during transcatheter ASD closure. Previous studies reported the rate of major complications as 1.6% in ASD patients receiving ASO8. In the current study, acute device embolisation was seen in four patients (one with CSO and three with ASO). All patients had large defects with either deficient or floppy septal tissues, which are reported risk factors for embolisation27,28. We therefore believed that the complications were related more to anatomy than to specific devices. We did not observe any case of delayed device embolisation after a mean follow-up of 25 months in the studied patient cohort.
The formation of thrombus, particularly on the left atrial disc, is another dreadful complication after ASD occlusion that could potentially lead to disabling stroke. Previous studies reported a lower risk of thrombus formation with the ASO as compared to other occluders such as the STARFlex®, CardioSEAL® (NMT Medical Inc., Boston, MA, USA) and PFO-Star® (Abbott Vascular, Santa Clara, CA, USA)29,30. Most of the thrombi were detected in the four-week follow-up TEE31. We did not observe any thrombus formation on the CSO in this study and no patient experienced any cerebrovascular event during subsequent follow-up. We believe the risk of thrombus formation should be very low in CSO because the CSO device is covered with a ceramic coating to lower even more the risk of thrombus formation.
Post-ASD occlusion tachyarrhythmias (atrial arrhythmias or SVT) are well documented4,22,32, and the estimated prevalence has ranged from 2-4%28,32. New-onset cardiac arrhythmia was detected in four patients receiving the CSO (1.95%) within one month after ASD closure. These arrhythmias were resolved after medical treatment alone. Arrhythmic complications were also seen in three patients receiving ASO. The development of atrial arrhythmia may be related to the inflammatory response triggered by the implantation of a foreign body33. High degree atrioventricular conduction blocks are even rarer (<1%) after ASD closure. Most of them occur during or within the first week after the procedure and most of them subside spontaneously. In our study, new-onset atrioventricular blocks were observed in three and four patients with CSO and ASO, respectively. One complete heart block was seen shortly after the procedure in a patient with ASO: the conduction disturbance resolved completely after one week of high-dose dexamethasone therapy.
Limitations of the study
A few limitations in the current study should be noted. First, there were varying lengths of time between transcatheter ASD closure and follow-up echocardiographic examination in this study. Moreover, the higher prevalence of acute residual shunt seen in ASO than in CSO patients may be biased by the fact that ASOs were used a few years ahead of the CSO. The initial lack of experience may have led to a less accurate choice of device size. Furthermore, the finding of a similar performance in both devices cannot be explained by patients having complex-type defects because these patients constituted less than 25% of all the cases. There was a limited follow-up duration (an average of 13 months) for patients receiving CSO implants and no definite conclusion could be drawn regarding the risks of late complications (e.g., late onset arrhythmia or device erosions). The procedural times were similar in both groups, but the CSO group had a shorter fluoroscopic time, probably due to the fact that the operators acquired the skills of ASD closure through previous AMPLATZER device implantation. Finally, the non-randomised design is another limitation of this study, and a larger, prospective, randomised study would be welcome to assess further the results seen in our study.
Conclusion
The Cera septal occluder is a safe and feasible device for transcatheter closure of atrial septal defects and it appears to be an attractive alternative to the AMPLATZER Septal Occluder because of its relatively low cost.
| Impact on daily practice The AMPLATZER Septal Occluder (ASO) has been the leading device for closing secundum-type atrial septal defects (ASD) over the last two decades. However, the high device cost prohibited its wide application in many parts of the world. This multicentre study compared the safety and efficacy of a new, less expensive, double-disc Cera septal occluder (CSO) to ASO. Between July 2004 and May 2012, 205 and 200 patients received CSO and ASO implants, respectively. The CSO group had similar ASD and device sizes, safety profile, prevalence of complex lesions (defect >26 mm or multiple defects), procedural time, implant success and complete closure rates as compared to the ASO group. The average equipment cost per patient was lower in the CSO group than in the ASO group. CSO can therefore be recommended as an alternative to ASO for transcatheter ASD occlusion. |
Conflict of interest statement
Y.Y. Lam is a consultant for St. Jude Medical, Boston Scientific and Lifetech Scientific Inc. The other authors have no conflicts of interest to declare.

