Introduction
The Carag bioresorbable septal occluder (CBSO; CARAG AG, Baar, Switzerland) is the first interatrial septal occluder with bioresorbable framework.
Methods
STUDY DESIGN
The study was a single-centre, prospective, single-arm trial. Ethics committee/regulatory approvals were obtained. ClinicalTrials.gov: NCT01960491.
DEVICE DESCRIPTION
The CBSO is the first occluder where the metal framework has been replaced with bioresorbable poly(lactic-co-glycolic) acid (PLGA) (Figure 1). The CBSO is self-centering, with two opposing polyester covers attached to a PLGA monofilament framework. The non-bioresorbable polyester fabric is secured to the monofilaments by platinum–iridium markers/sutures. A distal tip made of Phynox and a marker ring in the proximal filament holder ensure visibility under X-ray.
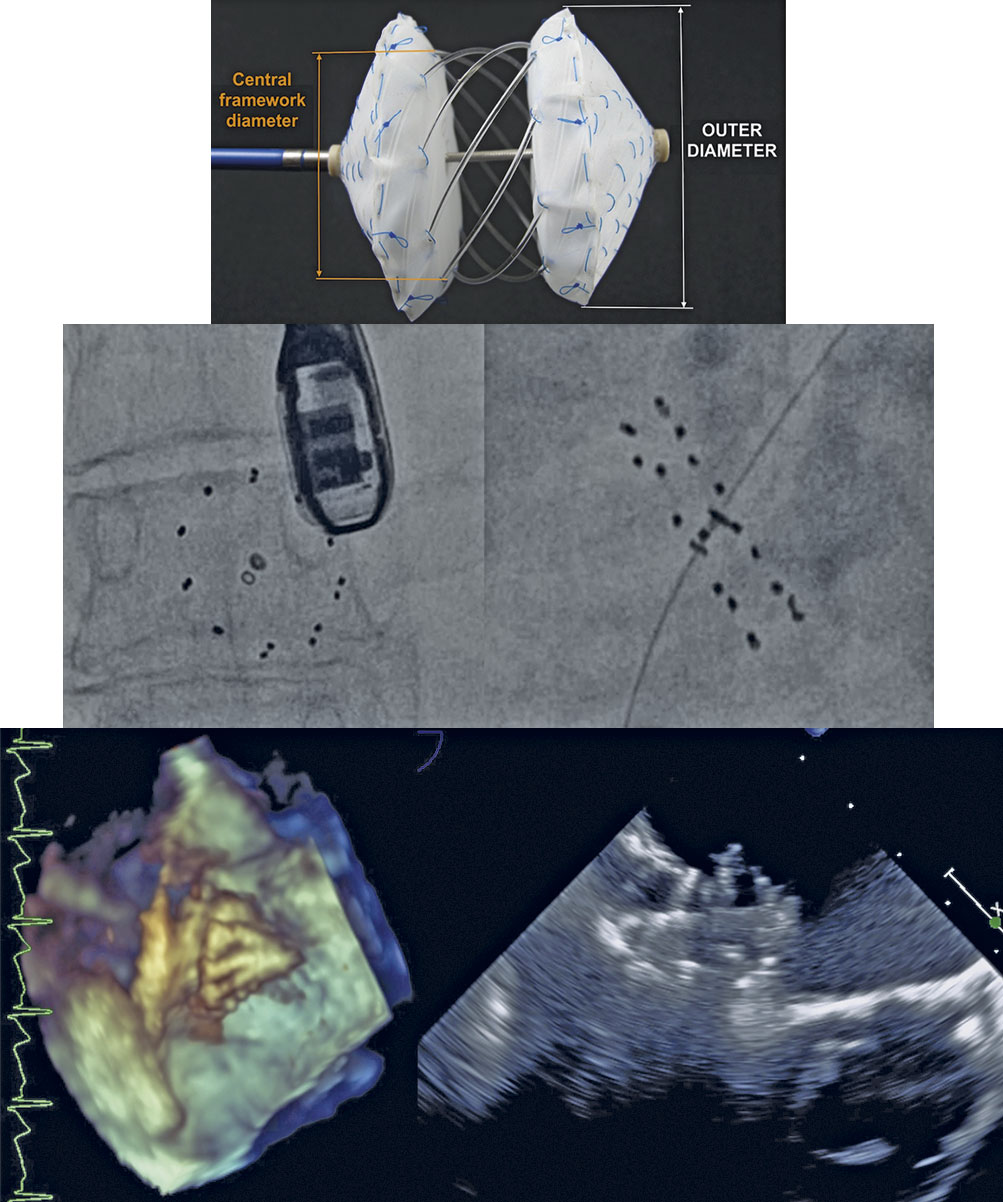
Figure 1. CBSO Occluder and fluoroscopic and echocardiographic visibility.
Delivery occurs over a 0.018” guidewire via a 12 Fr transseptal sheath through a delivery system allowing traditional deployment, right atrial side first or both sides in parallel. The over-the-wire device design allows reattachment and redeployment.
ENDPOINTS
The primary efficacy endpoint was clinically effective closure at six months assessed by transoesophageal echocardiography (TOE) including a bubble study/Valsalva manoeuvre for patent foramen ovale (PFO). The secondary endpoint was feasibility and safety including serious adverse events (SAEs) and evaluation of correct device placement during the procedure.
INCLUSION/EXCLUSION CRITERIA
Patients were required to have an isolated PFO or atrial septal defect (ASD) requiring treatment. ASD patients were required to show evidence of right ventricular overload, a stretched balloon diameter of the defect between 4-25 mm and adequate septal rim and septal morphology. PFO patients were required to have evidence of a cryptogenic stroke or transient ischemic attack (TIA) and contrast-echocardiographic evidence of a right to left shunt during the Valsalva manoeuvre.
POST-PROCEDURE REGIMEN
ASD and PFO patients received aspirin 100 mg/day for six months. PFO patients additionally received clopidogrel 75 mg/day for three months. Follow-up occured at discharge and at 1, 6, 12 and 24 months post-implantation.
PROCEDURE
All procedures were performed in line with the standard procedure for ASD/PFO closure under conscious sedation/local anaesthesia with TOE/fluoroscopic guidance. The device was disconnected from the delivery system allowing for reattachment through the guidewire and residual shunts detected using echo/fluoroscopy.
CLOSURE STATUS
For an ASD, complete closure was defined as no jet on colour Doppler, trivial leak <1 mm, small leak 1-2 mm, moderate leak 2-4 mm, and large leak >4 mm. Clinically effective closure for ASD is defined as complete closure or <2 mm leak. A PFO complete closure was defined as no bubbles on the Valsalva manoeuvre, trivial leak <5 bubbles, small leak 5-20 bubbles, moderate leak 20-40 bubbles and large leak >40 bubbles. Clinically effective closure for PFO was defined as complete closure or <5 bubbles on the Valsalva manoeuvre.
Results
Implantation success in 15/17 subjects (9 ASD, 6 PFO) had a procedural technical success rate of 88.2%. The average defect size of ASD patients was 16.5 mm (12.9-21.0 mm). The average PFO diameter was 8.0 mm (5.4-10.8 mm). The two failed implantations (one PFO and one ASD) occurred due to bad septal alignment. The average procedure time was 44.5 minutes skin-to-skin, with an average of 18 minutes of fluoroscopy.
FOLLOW-UP DATA
All patients were evaluated at discharge and at 1, 6, 12 and 24 months. In ASDs, 100% clinically effective closure at 12 months was maintained at 24 months. In PFOs, there was 50% clinically effective closure at 24 months with two small and one large residual shunt. No device deformation could be observed by transthoracic echocardiogram after 24 months.
SERIOUS ADVERSE EVENTS
No erosion, perforation, embolisation, myocardial infarction, stroke, TIA or interference with valvar apparatus occurred. One right atrial thrombus was resolved with anticoagulation. Two SAEs were deemed related to the device: palpitations (n=1) and thrombus (n=1).
Discussion
Implantation success was achieved in 15/17 patients (9 ASD, 6 PFO). Results in the ASD subset were excellent, with clinically effective closure in 100%. Clinically effective closure in the PFO subset was observed in 50% at 24 months, with two moderate shunts and one large shunt. Compared to the results reported for other PFO devices, these results are sub-optimal12, however, the number of patients is too small to draw any conclusions. Neither tunnel length, nor PFO size correlated with the closure rate. The compliance of septum primum may play a more important role with this device and perhaps using a larger device size may have led to better outcomes. The device may benefit from modifications to make a PFO-specific version.
Regarding SAEs, one patient developed a small right-sided thrombus. It was successfully treated with anticoagulants. Due to the limited number of subjects, a statistical calculation of a single event cannot be performed.
Study limitations
The study size is small. The inclusion criteria were restrictive but appropriate for a first-in-man (FIM) study. Based on current results, evaluation in a broader range of patients with ASDs is reasonable.
Conclusions
Based on the long-term results we conclude that the CBSO device is safe for use in ASD/PFOs, with no late complications observed. The CBSO appears to be highly effective for closure of small to medium ASDs. In PFOs the success rate was lower than expected. Larger studies for ASD patients to assess the safety and efficacy with large ASDs are planned. Further PFO studies, perhaps changing the implant strategy to use larger devices or modifying the current device are also desirable.
Funding
The CBSO FIM study was sponsored and funded by CARAG AG, Baar, Switzerland. The CBSO is now the “reSept ASD Occluder”, being developed by atHeart Medical AG, Baar, Switzerland.
Conflict of interest statement
H. Sievert reports fees from 4tech Cardio, Abbott, Ablative Solutions, Ancora Heart, Bavaria Medizin Technologie GmbH, BioVentrix, Boston Scientific, Carag, Cardiac Dimensions, CeloNova, Cibiem, Comed B.V., Contego, CVRx, Edwards Lifesciences, Endologix, Hemoteq, InspireMD, Lifetech, Maquet Getinge Group, Medtronic, Mitralign, Nuomao Medtech, Occlutech, pfm medical, ReCor Medical, RenalGuard, ROX Medical, Terumo, Vascular Dynamics, Vivasure Medical, Venus Medtech, and Veryan. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

