Abstract
Aims: To describe the initial experience with the GORE® septal occluder (GSO), a new septal occluder for the treatment of atrial septal defects (ASD) and patent foramen ovale (PFO).
Methods and results: This was a prospective single-centre review of patients undergoing percutaneous closure for a PFO or ASD with the GSO. A clinical evaluation and follow-up echocardiography were performed at three months with transoesophageal echocardiography (TEE) in case of PFO and transthoracic echocardiography (TTE) in ASDs. Between July 2011 and February 2012, thirty-eight patients underwent PFO (n=29) or ASD (n=9) closure with the GSO using TEE (n=36, 94.7%) or intracardiac echocardiography (n=2, 5.3%) guidance. In PFOs, three-month TEE was available in 24 patients and showed no residual shunt in 18 (75%), bubble shunt in two (8.3%) and bubble shunt after Valsalva in four (16.7%). In ASDs, three-month TTE showed no shunt in eight patients (88.8%) and residual shunt in one patient (11.2%). There was no device embolisation, air embolism, procedure-related stroke or pericardial effusion. No neurological events occurred during the follow-up period.
Conclusions: This initial experience with the new GSO device has demonstrated acceptable safety with no procedural complications and acceptable efficacy with low rates of residual shunting at three-month follow-up.
Introduction
Transcatheter closure of defects of the interatrial septum is the treatment of choice for secundum atrial septal defects (ASD) and is commonly used for patients with patent foramen ovale (PFO) and a history of cryptogenic stroke. The procedure has been associated with a low rate of complications and excellent short- and long-term results1,2.
The GORE® septal occluder (GSO; W.L. Gore & Associates, Flagstaff, AZ, USA) is a new septal occluder designed to treat both ASDs and PFOs. The device consists of a nitinol frame covered by ePTFE that has been engineered to facilitate rapid endothelialisation as well as to provide superior conformability, apposition and closure performance compared to the previous GORE® HELEX® device.
The objective of the present study was to describe the initial clinical experience with the GSO for the closure of both ASDs and PFOs in a tertiary care centre.
Methods
PATIENT SELECTION
Between July 2011 and February 2012, all consecutive patients with PFOs and all consecutive patients with ASDs in whom the balloon-stretched diameter multiplied by a factor of 1.5 was ≤30 mm (size of the largest GSO device) underwent implantation of a GSO device at the Montreal Heart Institute, Montreal, Quebec, Canada, and were therefore included in the study. The Montreal Heart Institute is a centre with a large experience in PFO and ASD closure with more than 400 procedures performed within the last four years. All clinical, procedural, echocardiographic and outcome variables were prospectively collected. Indications for ASD closure included evidence of right-sided chamber dilatation, and/or haemodynamic evidence of a significant left-to-right shunt confirmed by catheterisation3,4. Indications for PFO closure included a history of decompression illness or cryptogenic stroke confirmed by a neurologist. Patients with a history of cryptogenic stroke underwent the following investigations prior to device closure: carotid Doppler, Holter monitor, thrombophilia screen and transthoracic and transoesophageal echocardiography (TTE and TEE). All these investigations were performed by the neurologist prior to patient referral for PFO closure. Diagnosis of patent foramen ovale was defined as the existence of spontaneous right-to-left shunt or the presence of ≥10 micro bubbles in the left atrium after intravenous injection of agitated saline at either rest or post Valsalva5.
The Director of Professional Services at the Montreal Heart Institute approved the review of medical charts and documentation. This was a single-centre experience with the GSO that has not been solicited or financed by any company. The motivation to perform this study was based on the desire to evaluate clinically this new device.
DEVICE IMPLANTATION
The GSO device is composed of a platinum-filled nickel-titanium (nitinol) wire frame covered with expanded polytetrafluoroethylene (ePTFE). The wire frame is formed from five wires, shaped into the right and left atrial discs, the eyelets and the locking loop (Figure 1). There are four available GSO sizes (15, 20, 25 and 30 mm). The delivery system of the GSO allows precise positioning with a single handle control (Figure 1), and possesses a retrieval cord allowing device removal after the initial release in case of an unsatisfactory result. Although the GSO might be seen as a modification of the HELEX, the main changes in the design of the device lead to the conclusion that this is a new device rather than a modification of the HELEX system. Briefly, the main variations between the GSO and the HELEX device are the following: 1) five-wire occluder frame design versus one; 2) increased conformability and septal apposition provided by a multi-petal disc design versus a helical disc design; 3) platinum cored nitinol wire frame versus solid nitinol; 4) delivery handle versus no delivery handle; and 5) increased porosity ePTFE versus less porous ePTFE.
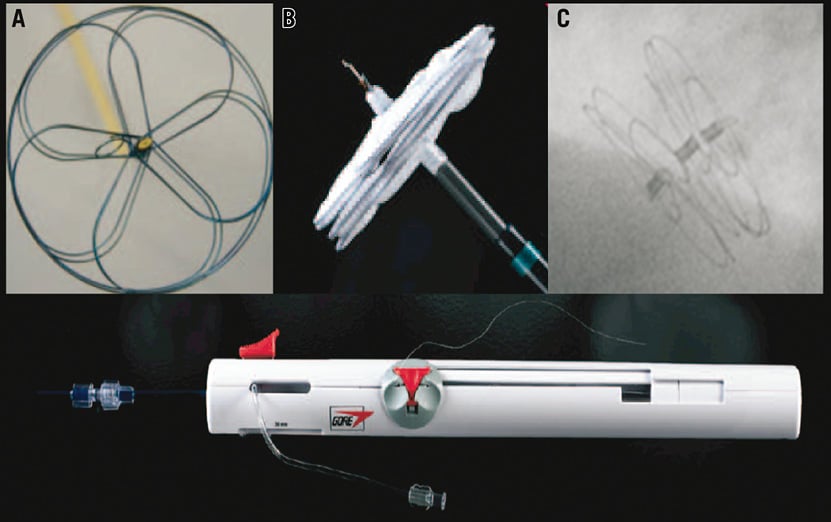
Figure 1. Multiple views of the GORE® septal occluder. Frontal view of the five-wire frame (A), lateral view of the device attached to the delivery system (B), and fluoroscopic imaging showing the flat apposition of the device after deployment (C). Gore septal occluder delivery handle (lower image).
The procedure was performed under general anaesthesia or mild sedation depending on the use of TEE or intracardiac echocardiography (ICE) guidance, respectively. The use of echocardiographic guidance for all ASD and PFO closures regardless of the device is the standard of care in our institution. All procedures were performed using intravenous unfractionated heparin (60 IU/kg) to obtain an activated clotting time (ACT) >250 seconds. Venous access was obtained in the right or left femoral vein using a 12 Fr sheath. Subsequently, a 0.032-inch J-tip wire was advanced across the defect into the left upper pulmonary vein and exchanged for a stiff guidewire using a 6 Fr multipurpose catheter. A sizing balloon inflated to stop-flow was used to assess the dimensions of the defect and the presence of additional defects.
In the case of ASD closure, device selection was based on the balloon-stretched diameter multiplied by a factor of 1.2-1.5. GSO implantation was not recommended and therefore not performed in ASDs >20 mm. For PFO closure, the choice of device size was based on more factors, namely balloon-stretched diameter, tunnel length and compliance, as well as the septal thickness and the presence of interatrial aneurysm.
The device was positioned and deployed using both fluoroscopic and echo guidance. In the case of PFO closure, patients underwent a bubble study immediately post device deployment to assess residual shunting at rest and with Valsalva.
All patients were treated with dual antiplatelet therapy (aspirin 80 mg/day and clopidogrel 75 mg/day) for a minimum of three months, followed by an additional nine months of aspirin. Those patients treated with warfarin prior to the procedure resumed therapy post procedure with the addition of clopidogrel for three months.
ECHOCARDIOGRAPHY
Transoesophageal echocardiography (iE33 ultrasound system and X7-2t matrix array transducer; Philips Healthcare, Andover, MA, USA) or ICE (St. Jude Medical, Minneapolis, MN, USA) was used for guiding the intervention. Atrial septum aneurysm (ASA) was defined as a movement of the septum ≥10 mm with an aneurysm diameter ≥15 mm6,7. A tunnel PFO was considered when there was at least 10 mm between the flap of the valve and the septal wall of the PFO7. Deficient aortic rim was defined in case of <5 mm8. Echocardiography was also used to evaluate the final result of the procedure. For ASDs, the final result was assessed by colour Doppler signal whereas PFOs were evaluated using a bubble study with agitated saline. Closure of the PFO was assessed during the five beats following injection of agitated saline, and success was defined as passage of fewer than five bubbles from the right to the left atrium7. A TTE was performed in all patients the day after the procedure in order to assess the position of the device, the degree of residual shunt and the presence of pericardial effusion.
FOLLOW-UP
A clinical evaluation was performed by the treating physician three months after the index procedure. Follow-up echocardiography was performed at three months with TTE in case of ASDs and TEE in PFOs. In patients with PFOs, residual shunting was assessed using intravenous injection of agitated saline at rest and post Valsalva and classified as previously described.
STATISTICAL ANALYSIS
The results are expressed as mean±standard deviation (SD) for normally distributed data. Comparisons between groups were performed using the chi-square or Fisher’s exact test for categorical variables. Results were considered statistically significant at a p-value <0.05. Statistical analyses were carried out using SPSS package v16.0 (SPSS Inc., Chicago, IL, USA).
Results
PATIENT CHARACTERISTICS
During the study period, 38 consecutive patients underwent implantation of a GSO for either PFO (n=29, 76.3%) or ASD (n=9, 23.7%). Patient characteristics are shown in Table 1. All patients with PFOs presented at least one episode of paradoxical cerebrovascular accident. The indication for ASD closure was right ventricular volume overload in six patients (66.6%) and the presence of a significant shunt (QP/QS >1.5) without right ventricle enlargement in three (33.3%).
DEVICE IMPLANTATION
In patients with PFOs, the most common GSO size was the 25 (n=15, 51.7%) followed by the 20 (n=10, 34.5%), whereas in ASDs the 25 (n=4, 44.4%) and 30 (n=4, 44.4%) were the most frequently used (Table 2). Mean fluoroscopic time was 6.9±2.7 min for PFOs and 12.7±6.6 min for ASDs. The procedure was guided by TEE and ICE in 36 patients (94.7%) and two (5.3%), respectively. In one PFO patient with a non-compliant tunnel that could not be “detunnelised” with the sizing balloon, transseptal puncture was performed in order to achieve a better apposition of the device. In one patient with multiple ASDs, two devices (30 and 20) were implanted with a good result. Although procedural success was achieved in all patients with PFOs and ASDs, the initial device was retrieved and replaced by a new one in four patients for the following reasons: 1) unsatisfactory closure in one patient with ASD and ASA (new device upsized from 20 to 25); and 2) inadequate position of the locking loop after releasing the device in three patients (Figure 2). In these three patients the device was removed with the retrieval cord and a second GSO was successfully implanted. There were no cases of device embolisation, relevant air embolism or procedure-related stroke. None of the patients experienced a significant vascular access-related bleeding or complication. No new pericardial effusion was observed during the procedure. No significant clinical events or arrhythmias were documented during hospitalisation and all patients were discharged the day after the index procedure.
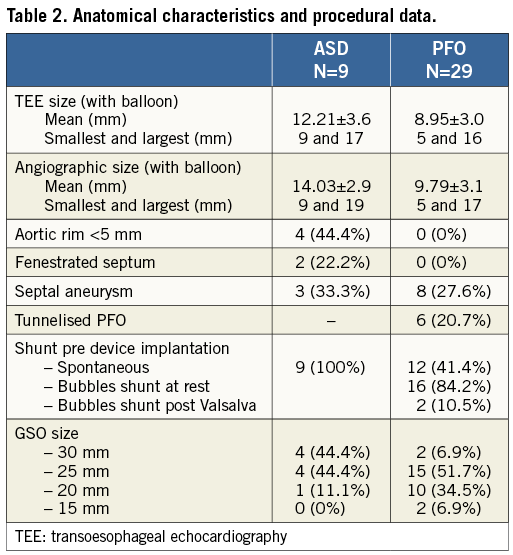
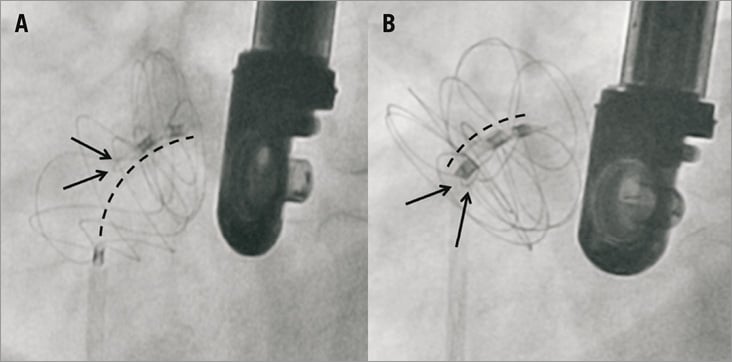
Figure 2. Inadequate (A) and successful locking (B) of the GSO after the initial deployment. A) shows a patient with inadequate locking of the two discs after the initial deployment. This problem can be easily detected by the presence of the locking loop (arrows) between the right and the left disc and a distance >15 mm between the proximal and the distal markers (line). At this point, the device can be easily removed using the retrieval cord and a new device can be implanted. B) shows the successful locking of the second device in the same patient. In this case the wire loop locked the two discs (arrows) and the distance between markers remained <15 mm.
POST-IMPLANTATION RESULTS IN PFO
In patients who underwent PFO closure, a bubble test was performed after successful implantation of the device (n=27). Eighteen patients (66.7%) presented no residual shunt, while three patients (11.1%) had a shunt at rest and six (22.2%) had a shunt with Valsalva. A residual shunt with colour Doppler was observed after device implantation in one of the two patients guided by ICE. Of note, the patient presented a giant ASA. At 24 hours, TTE showed no residual shunt in 28 patients (96.6%) and only a mild residual shunt (3.3%) in the patient with the giant ASA. None of the patients presented device embolisation, thrombus formation or pericardial effusion.
POST-IMPLANTATION RESULTS IN ASD
In ASDs, intraprocedural TEE showed complete sealing in three (33.3%) and a mild peri-device shunt in six (66.6%). Transthoracic echocardiography 24 hours after GSO implantation showed no residual shunt in eight patients (88.8%) and mild residual shunt in one (11.2%). None of the patients presented device embolisation, thrombus formation or pericardial effusion.
FOLLOW-UP
Clinical and echocardiographic follow-up was performed three months after the index procedure. None of the patients presented a late device embolisation, device thrombosis, documented arrhythmias or pericardial effusion.
FOLLOW-UP OUTCOMES IN PFO
No recurrent paradoxical strokes or peripheral emboli were documented. A TEE at three months was available in 24 patients (89%). TEE showed no residual shunt in 18 (75%), shunt at rest in two (8.3%), and shunt after Valsalva in four (16.7%). Four patients (16.6%) with a positive shunt post procedure presented no shunt at three months, in two (8.4%) the shunt improved from rest to post Valsalva, in two (8.4%) the shunt persisted at the same degree, and in two (8.4%) the shunt increased (from absent to post Valsalva in one and from post Valsalva to rest in the other). In patients with residual shunt at either rest or Valsalva, the prevalence of ASA was 50%, whereas in those without shunt it was 22.2% (p=0.195). All patients with residual shunt were scheduled for a second TEE at one-year follow-up (results not available as all implants were performed during the last year).
FOLLOW-UP OUTCOMES IN ASD
Transthoracic echocardiography was available in all nine patients (100%) at three months. Only one patient (11.1%) presented residual flow by Doppler. In this patient, the septum was fenestrated and the size of the aortic rim was 5.4 mm. There was evidence of right ventricular remodelling at three months in two patients (33.3%), and three of the four patients (75%) with dyspnoea (NYHA ≥2) experienced a clinical improvement after ASD closure.
Discussion
This initial clinical experience with the GSO has illustrated the safety of this device in clinical patients being treated for an ASD or PFO. There were no short- or mid-term complications such as mortality, stroke, air embolisation, infection, device embolisation, thrombus formation on the device, arrhythmia or vascular access complications. Such low rates of short- and mid-term complications have already been described in previous publications with other devices, in particular with the AMPLATZER® septal occluder device7,9,10 (AGA Medical Corporation, Golden Valley, MN, USA).
PATENT FORAMEN OVALE CLOSURE
This experience demonstrates the high efficacy of the GSO device for PFO closure. In the patients treated there were low rates of residual shunt at three months (25%). Importantly, the GSO device provided immediate and adequate sealing post deployment in the majority of patients, illustrating a good mechanical closure immediately post implantation. Although the TEE criterion for complete closure was the presence of a shunt <5 bubbles, all patients classified as having a complete closure post deployment or at follow-up did demonstrate presence of a shunt. As previously described11, the presence of ASA may play an important role in the closure success as it was documented in 50% of patients with residual shunt at follow-up. Of note, the only patient with residual Doppler shunt at three months presented a major reduction of the flow through the PFO, but the presence of a giant and hypermobile ASA was probably the main reason for not achieving an optimal result11.
Clinically, none of the patients presented with recurrent stroke during the follow-up period. Although the absence of long-term follow-up and the relatively small sample size constitute the main limitations for extracting major conclusions on the efficacy of the device for preventing stroke, it is important to point out that no air embolisation occurred during implantation and no thrombus formation on the device was observed during follow-up. In addition, the low rate of immediate and three-month residual shunt may indicate a low risk of recurrent cerebrovascular events during long-term follow-up12.
ATRIAL SEPTAL DEFECTS CLOSURE
In ASDs, closure rates were high (89%), with only one patient presenting residual flow at three months. These results were similar to previous publications using the AMPLATZER septal occluder as residual flow ranged between 0.9% and 19.2%5,13,14 and appeared to be lower compared to the 25% with STARFlex (NMT Medical, Boston, MA, USA) at six months13 and the 24.8% with HELEX at 12 months15.
In our series, the maximum ASD diameter that was attempted and successfully closed was 19 mm. Sizing recommendations for the GSO are still being developed but, given the five-petal design and increased coverage provided by the device, we tend to follow a strategy of 1.5:1 device to defect sizing for optimal closure. The largest available GSO device is 30 mm. Therefore, a defect of 18-19 mm with adequate rims can be safely closed with this device.
Interestingly, 22% of patients presented a fenestrated septum and 44% no aortic rim. All the patients with deficient aortic rim had successful closure at follow-up. Concerns of device erosion have been discussed in detail at the recent FDA panel on ASD closure devices. It is well documented that use of the AMPLATZER ASO device has been associated with erosions in patients with insufficient rims and this is now considered a contraindication for this device. In theory, the softer and more conformable design of the GSO should lessen concerns of erosion with this device; however, further long-term studies with the device will be required. In fact, there have been no reported device erosions with the HELEX device, a similar device to the GSO.
The only patient with residual shunt among those treated for ASD had a fenestrated septum that did not allow for complete sealing with a single device. The GSO, however, has been designed to close both standard secundum defects as well as fenestrated atrial septums by using a central pin rather than a device waist. The enhanced radial force between the two discs provides improved closure performance in case of multiple adjacent defects. In our limited experience treating two patients with a fenestrated septum, one had complete closure with multiple devices16 while the other did have a residual shunt.
TECHNICAL ASPECTS
The GSO device is a simple design that permits precise positioning with a single handle control (Figure 1). The handle permits one-handed device deployment and provides the operator with the ability to recapture the device and reposition it easily if the position is not optimal16. The device can be pulled firmly against the septum to ensure adequate contact prior to deploying the right-sided disc. Once an adequate position has been confirmed, the device is deployed using the release system; however, the device remains attached to the delivery system by way of the retrieval cord. At this point, the operator can evaluate the final position and orientation of the device without tension from the delivery catheter, which has been completely released. The change in position and orientation may be crucial as non-covered defects may be covered or vice versa. This point constitutes one of the main advantages of the GSO system compared to other devices, since the operator still has the possibility to remove the device by using the retrieval cord and implant another device in case of an unsatisfactory result. In case of device retrieval, recrossing of the defect is however required in order to implant a new device.
Despite the fact that the present series constitutes the initial experience using the GSO device in our institution, we observed a very fast learning curve, as depicted by the short fluoroscopic times. The mean fluoroscopic time in patients with PFOs was 6.9 min which contrasted with the 10, 8 and 8 minutes observed in a highly experienced centre with the AMPLATZER septal occluder, HELEX and Premere (St. Jude, Minneapolis, MN) device, respectively7. We systematically utilised a short 12 Fr femoral sheath to implant the device. Although we did not have any vascular complications, the required size of the sheath (11 or 12 Fr) might increase the potential risk of vascular complications and may represent a limitation for its usage in some paediatric patients. We were required to remove the device in three patients in whom the device was not optimally locked. Occurrence of this phenomenon is not hazardous as it is easily detected after the initial deployment by the presence of a distance >15 mm between the markers of the discs and the lack of stability of the right atrium disc (Figure 2). In addition, the system is always held by the cord and is therefore easily retrievable. In our series, the device was successfully retrieved in all three patients and a new GSO was deployed without incident after neutralising the tension of the delivery catheter.
Study limitations
The results of the present study must be interpreted in context, as this was an observational study with a relatively small number of patients, incomplete TEE follow-up in 10% of the PFO patients and no comparison with other available devices. The aim of the present paper was to describe our initial experience with the GSO as well as to provide data that operators might find useful when using this device.
Conclusions
In conclusion, the present study describes our initial experience with the new GSO device for the closure of PFOs and ASDs. Clinical results demonstrated safety with no periprocedural complications, and efficacy with an acceptable rate of residual shunts immediately post implantation and at three-month follow-up. Further data with a larger number of patients and longer follow-up will be necessary to confirm these results, to evaluate the occurrence of device thrombosis and to compare the closure performance with other available devices.
Conflict of interest statement
R. Ibrahim is a consultant for St. Jude Medical and Gore Medical. A. Asgar is a consultant for Gore Medical. The other authors have no conflicts of interest to declare.

