Abstract
Aims: To compare the mid-term efficacy and safety of the bioabsorbable BioSTAR® device with the non-bioabsorbable CardioSEAL® device for percutaneous patent foramen ovale (PFO) closure.
Methods and results: All 81 consecutive patients who underwent PFO closure with the CardioSEAL® or BioSTAR® device between June 2003 and July 2008 were included. The presence of a residual shunt (minimal, moderate or large) was measured in both groups at six months follow-up, using contrast transthoracic echocardiography. Forty-four patients (48.4±11.4 years) received the CardioSEAL® device and 37 patients the BioSTAR® device (47.9±10.7 years). There were no significant differences in short-term complications. Two patients who received the BioSTAR® device developed a recurrent transient cerebral ischaemic event. Overall, atrial arrhythmias occurred in 19%, with no difference between both groups. At six months, a residual shunt was present in 29% (27% minimal, 2% moderate) using the CardioSEAL® device compared to 28% (17% minimal, 11% moderate) using the BioSTAR® device (p=0.18). A predictor for residual shunt could not be found.
Conclusions: There is no difference in safety and efficacy at six months between the CardioSEAL® and BioSTAR® device used for PFO closure. However, using the BioSTAR® device tends to be associated with a higher percentage of moderate shunting.
Introduction
A patent foramen ovale (PFO) has been associated with paradoxical embolic events such as cryptogenic stroke, peripheral embolism and decompression illness in divers, especially in young adults1-3. These patients are at increased risk of recurrent thromboembolic events, despite the use of anticoagulation or antiplatelet therapy4-6. Moreover, patients with an atrial septum aneurysm (ASA) have a higher risk of stroke recurrence7-9. Since the initial report in 199210, percutaneous PFO closure has been used with increasing frequency and has shown promising results regarding safety and efficacy. Various occlusion systems have been used, with different complication and success rates11-14. A new bioabsorbable device (BioSTAR®; NMT Medical, Boston, MA, USA) has been developed to avoid potential problems such as thromboembolism, erosion and inflammation which have been attributed to permanent synthetic implants. The BioSTAR® device consists of a totally biodegradable matrix made of a porcine intestinal collagen layer, mounted on a nitinol framework. Initially, promising results were shown in the first in human trial15. However, a high rate of residual shunting was noticed at short-term follow-up16. Another self-expanding, double umbrella device mounted on the same framework is the CardioSEAL® device (NMT Medical, Boston, MA, USA). This non-bioabsorbable, permanent device is widely used and associated with a low incidence of complications and recurrent thromboembolic events17,18. Both devices are shown in Figure 1.

Figure 1. The BioSTAR® device (left) and the CardioSEAL® device (right).
The aim of our study is to compare these two devices in patients with presumed paradoxical embolism undergoing percutaneous PFO closure with respect to periprocedural and mid-term complications, the recurrence of paradoxical embolism and the efficacy of PFO closure.
Methods
Study population
All 81 patients who underwent a PFO closure in our centre using the CardioSEAL® or BioSTAR® device between between June 2003 and July 2008 were included. During that study period 87 PFO closing procedures were performed. In six patients, an Amplatzer® PFO occluder (AGA Medical, Golden Valley, MN, USA) was used and these patients were excluded. Between June 2003 and October 2007 the CardioSEAL® device was used in 44 patients. From November 2007 until July 2008 the BioSTAR® device was used for PFO closure in 37 consecutive patients. A PFO was identified by standardised contrast transthoracic (cTTE) using second harmonic imaging or contrast transesophageal echocardiography (cTEE) with spontaneous or provokable right-to-left shunt after injection of 10 ml of agitated saline in an antecubital vein. An atrial septum aneurysm was defined as an excursion of the interatrial septum of at least 10 mm. The study was approved by the local ethical committee.
Closing procedure
As described previously, closure of the PFO was performed under general anaesthesia and TEE monitoring in all patients who received the CardioSEAL® device and in the first six patients who received the BioSTAR® device16,19. Thereafter, the BioSTAR® device was implanted under local anaesthesia using intra-cardiac echocardiographic (ICE) guidance. Concomitant biplane fluoroscopic guidance was used in all patients. All patients were treated with antiplatelet therapy prior to the closure procedure. A bolus of 5000 U of heparin was administered after accessing the right femoral vein and each patient received an intravenous prophylactic dose of antibiotics at the time of the procedure. The left femoral vein was used in case of ICE-guiding. The PFO was passed using a standard multipurpose catheter and exchange wire. After thoroughly flushing to prevent air embolism, the loaded implantation system was advanced across the atrial septum and the device expanded and released under fluoroscopic and echocardiographic guiding. Within 24 hours after closure, an electrocardiogram, chest X-ray and cTTE were performed. All patients were discharged on aspirin 100 mg once a day for a period of six months and clopidogrel 75 mg once a day during one month. Patients on oral anticoagulant therapy before the procedure were discharged on a combination of oral anticoagulant therapy and clopidogrel for one month. Endocarditis prophylaxis precautions were recommended for six months.
Successful device implantation was defined as completion of the procedure without the occurrence of major events (death, device embolisation, device malpositioning with replacement or need for surgical intervention).
Complications and outcome
All procedural complications, immediately related to the procedure within six months, were reported. Complications were divided into major and minor complications according to the classification scheme of Khairy et al20. According to this review article, major complications include haemorrhage requiring blood transfusion, occurrence of cardiac tamponade, need for procedure related surgical intervention, massive fatal pulmonary emboli and death, related to the closing procedure. Minor complications were defined as device malpositioning with successful catheter repositioning, bleeding not requiring blood transfusion, occurrence of new onset atrial arrhythmias (atrial flutter or fibrillation), transient atrioventricular block, device arm fractures, device embolisation with successful catheter retrieval, asymptomatic device thrombosis, need for re-catheterisation, transient air embolism, transient ST-segment elevation, femoral arteriovenous fistula formation, femoral haematoma, and other minor complications related to the closing procedure.
Clinical information was obtained by an out-patient visit to a cardiologist at six months.
New-onset supraventricular tachyarrhythmias (SVT) were diagnosed by a 12-lead electrocardiogram or Holter monitoring in patients without a history of SVT at baseline. The history of recurrence of stroke or TIA was confirmed by a neurologist using the appropriate imaging techniques.
Efficacy
The routine follow-up program for the CardioSEAL® device group included TTE without contrast at 24 hours and cTTE at six months after device implantation. The group who received the BioSTAR® device had a cTTE at 24 hours and at six months, and an additional cTTE one month after implantation. All echocardiographic examinations six months after device implantation were reviewed by two independent physicians. The efficacy of PFO closure was based on the residual shunt. Micro bubbles were counted in the left atrium within three cardiac cycles after right heart opacification. Residual shunting was categorised as follows: small shunt (<30 bubbles in the left atrium), moderate shunt (30-100 bubbles in the left atrium) and severe shunt (>100 bubbles in the left atrium). Recently, an excellent interobserver variability has been demonstrated using cTTE for right-to-left shunt detection21. All cTTE examinations were performed using second harmonic imaging22.
Statistical analysis
Descriptive statistics were used to describe patients’ characteristics. Continuous variables with normal distribution are presented as mean ±standard deviation. Univariate statistical analysis was used to identify risk factors for residual shunting after PFO closure. All statistical analyses were performed by using SPSS software (version 14.0 for Windows).
Results
Patient population
Baseline and PFO characteristics of the patient population are listed in Table 1.
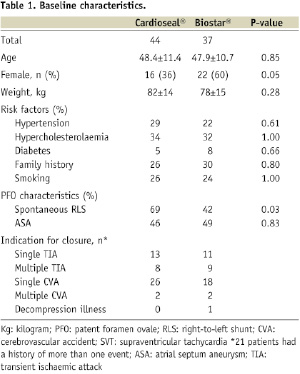
In 44 patients PFO closure was performed with the CardioSEAL® device. Thirty-six percent were female with a mean age of 48.4±11.4 years. In the BioSTAR® device group, 60% were women with a mean age of 47.9±10.7 years. An ASA was detected in 46% and 49% in the CardioSEAL® device group and in the BioSTAR® device group, respectively. All patients in the CardioSEAL® device group underwent PFO closure because of cryptogenic stroke or TIA. In the other group, one patient was treated because of decompression illness. Twenty-one patients (26%) had a history of more than one thromboembolic event.
Periprocedural complications
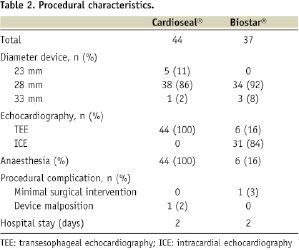
The implantation of the CardioSEAL® device was successful in 98% of the patients. In one patient a 28 mm device was malpositioned and successfully replaced by a 33 mm device. A 28 mm device was delivered in 86% of the patients in this group. One patient developed a minimal groin haematoma immediately after the procedure not requiring a blood transfusion nor surgical intervention. There were no other in-hospital complications.
In the BioSTAR® device group, 36 patients (97%) had a successfully device delivery and deployment. In one patient the device was pulled trough the PFO before it was released, but could not be recovered into the sheath. Therefore, surgical exploration of the femoral vein was necessary to retrieve the device. This patient successfully received an Amplatzer® Septal Occluder device one month later. A 28 mm device was implanted in 92% of the patients in this group. Four patients (11%) developed a minimal inguinal haematoma. The closure characteristics are summarised in Table 2.
The in-hospital complications are shown in Table 3.
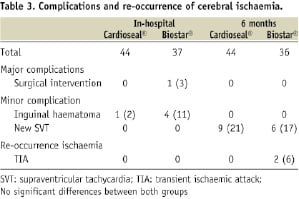
Mid-term complications and outcome
Within six months after closure, no major complications occurred in either of the two groups. In the CardioSEAL® device group, nine patients (21%) developed a SVT. Seven patients were treated successfully with antiarrhythmic drugs, two patients needed electrical conversion. No other complications occurred in this group. No re-occurrence of stroke or TIA was reported.
In the BioSTAR® device group, six patients (17%) experienced a new paroxysmal SVT, one patient needed electrical cardio version, three patients were treated medically, the other two patients had a transient atrial tachycardia which resolved spontaneously. No predictor for the development of SVT after PFO closure could be identified by using univariate analysis. A 51-year-old male patient developed a TIA within one month after closure with the BioSTAR® device. This patient had a residual shunt at that time and an atrial septum aneurysm on baseline echocardiography. A 50-year-old female patient suffered from a recurrent TIA, six months after closure in the absence of a residual shunt on cTTE (Table 3).
Efficacy
At six months follow-up, complete closure was present in 71% of the patients who received the CardioSEAL® device and in 72% of the patients who received the BioSTAR® device (p=0.18). In the CardioSEAL® device group, 27% had a trivial shunt and 2% had a moderate shunt, compared with 17% and 11% respectively in the BioSTAR® device group. No large shunts were detected. The efficacy data are shown in Figure 2.
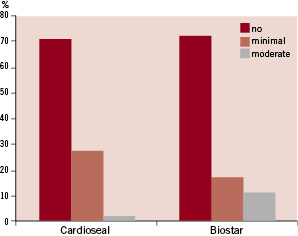
Figure 2. Percentage of residual shunting diagnosed by cTTE at six months follow-up.
Combining moderate and large shunts results in a non-significant higher percentage of residual shunt in the BioSTAR® device group (11% versus 2 %, p=0.17). No predictor for the presence of a residual shunt at six months follow-up could be identified using univariate analysis.
Discussion
In the past, several occluder systems have been used for transcatheter PFO closure13,14,23. They all have different designs and consists of a synthetic matrix, which is encapsulated by fibrous tissue over time. Inherent risks include device embolisation and device fracture, infection, erosion, thrombus formation and arrhythmias24-26. Therefore, a bioabsorbable closure device has been introduced recently. It is associated with the ability to induce a host connective tissue response and should lead to a more rapid and complete neo-endothelialisation. The collagen matrix is gradually resorbed over a period of about two years, leaving only the frame behind27,28. The non-absorbable CardioSEAL® device, used in our study, is constructed from a knitted Dacron® fabric, mounted on the same low profile nitinol framework as the bioabsorbable device. We accomplished the first “head-to-head” comparison of these two devices in a single centre setting.
Complications
The rate of complications of PFO closure with the CardioSEAL® device is described in several studies. The periprocedural major complication rate varies between 1.6 and 4.6%29,30. We found a periprocedural complication rate of 2% in the CardioSEAL® device group. Recently, Taaffe et al described a randomised comparison of the CardioSEAL® device with the Amplatzer® and Helex® device23. They examined 660 patients, with 220 patients per group and found more thrombus formation (3.6%) and atrial fibrillation (4.5%) one month after the procedure, in the group who received the CardioSEAL® device. Anzai et al showed that the CardioSEAL® device is more likely (22%) to have thrombus formation than the Amplatzer® device, using cTEE31. We found a short-term complication rate of 21% in the CardioSEAL® group, all related to supraventricular arrhythmias. We did not detect any thrombus in the CardioSEAL® group, realising that thrombus assessment with TTE only might be not revealing.
In the BEST trial, 58 patients (54 PFO, 4 ASD) were treated with the BioSTAR® device15. In two patients (3%), the device was malpositioned. In one of them a larger device was introduced and in the other patient the defect was closed with an alternative device. Furthermore, they described no major adverse events during follow-up. Five patients (8.6%) were treated for supraventricular arrhythmia, one patient developed urticaria and in one patient a mobile echogenic mass was seen on the right atrial side of the device, which resolved after anticoagulation therapy. As previously reported, we had a procedural complication in one patient (3%)16. In our series, 17% developed a new transient SVT, no other complications occurred during mid-term follow-up. Comparing the BioSTAR® device and the CardioSEAL® device, no significant differences could be observed regarding periprocedural, short- and mid-term complications. However, quite a high percentage of new SVT is seen in both groups. On the other hand, there seems to be a trend towards a higher percentage of inguinal haematoma (11% versus 2%) in the BioSTAR® device group, probably due to the extra access site using ICE. In two reports which compared PFO closure guiding with ICE and TEE, no differences could be found regarding safety32,33.
Re-occurrence of thromboembolic events
A recent report showed an annual re-event rate of 0.9% for stroke and a combined annual event rate for stroke and TIA of 3.4% in 216 patients treated with the CardioSEAL device. Interestingly they found that 30% of the patients with a recurrent event had clear evidence of pathology unrelated to a cardio-embolic source. In our CardioSEAL® group, no recurrence of stroke or TIA did occur within six months after PFO closure.
In the BEST trial, no thromboembolic events were noticed after six months follow-up15.
We report two patients (5.6%) with symptoms of recurrent TIA in the BioSTAR device group. Previous studies support the hypothesis of the increased risk of re-events in the presence of a residual shunt and/or ASA7,8. One patient indeed had a residual shunt and an ASA. In the other patient PFO closure was achieved and confirmed by cTTE and no thrombus was seen on the device. It may be presumed that the cause of recurrent TIA might be other than paradoxical embolism.
Residual shunt
The presence of a residual right-to-left shunt after PFO closure is widely described in literature. Recently, Wahl and Meier addressed that complete PFO closure is achieved in 51-100% of patients, using a variety of devices35. According to this review paper, complete PFO closure at six months using the CardioSEAL® device varies between 51% and 89%8,17,18,30,36. Braun et al reported a residual shunt, using cTEE, in 28% of the patients after one month and in 20% of the patients after six months, using the CardioSEAL® device28. At six months after the procedure, we found an overall residual shunt rate (including small shunts) of 29% for the CardioSEAL® device. Only 2% of the patients had a moderate shunt and no large shunts were detected.
The BEST trial showed a residual shunt rate of 8% at one month and of 4% at six months, using contrast TTE. Successful defect closure was defined as procedural success with no shunt or trivial (<10 bubbles) shunt, so only moderate and large shunts were reported15.
In our series, a residual shunt rate of 28% was noticed at six months follow-up in the BioSTAR® group. We earlier reported a residual shunt rate of 45% (minimal 30%, moderate 12%, severe 3%) in 33 patients, one month after the implantation of the BioSTAR® device16. Comparison of our results with the results of the BEST trial is difficult concerning the difference in shunt grading. When we only count moderate and large shunts, a residual shunt rate of 15% at one month and of 11% at six months was achieved.
Overall, a comparable closure rate is seen between the CardioSEAL® and the BioSTAR® device. However, more moderate shunts were detected (11% versus 2%) using the BioSTAR® device. A hypothesis for the difference in residual shunting is that in some patients, a mechanical occlusion of the defect with a synthetic device might result more rapidly in defect closure compared to the more natural healing process using the BioSTAR® device. This is in contrast to the findings of Jux et al, who showed a significantly more thorough coverage of the device by tissue in a sheep model27. Maybe there is an inter-individual difference regarding the formation of neo-endothelium and granulation tissue in response to the BioSTAR® device.
Limitations of the study are the non-randomised, retrospective design, the single-centre characteristics and the small number of patients. Regarding complications, we must stress that we only performed TTE during follow-up, which is less sensitive for thrombus detection on the devices.
In conclusion, our study shows that percutaneous PFO closure can be achieved safely with the CardioSEAL® device and with the BioSTAR® device. No significant differences could be revealed regarding implantation success, periprocedural, short-term and mid-term complications. The efficacy of closure is comparable, however the use of the BioSTAR® device is associated with a higher percentage of moderate shunting. Larger, randomised trials are necessary to determine the optimal closure device in this patient population.

