
Percutaneous catheter-based closure of atrial septal defects (e.g., patent foramen ovale [PFO]) has been performed for more than 40 years and preceded percutaneous coronary interventions (PCI) by a couple of years1. PFO closure was first described as a specific structural intervention in adult cardiology 26 years ago2. The clinical introduction of the AMPLATZER™ Septal Occluder (Abbott, Plymouth, MN, USA) on 10 September 1997, 21 years ago, turned this procedure into what has been the easiest intervention in adult cardiology with probably the best net yield ever since3.
The thought of a permanent implant into the heart in a predominantly preventive procedure (therapeutic exceptions are PFO closures for migraine, platypnoea orthodeoxia, exercise desaturation, and sleep apnoea) is not overly appealing. Hence, the adoption of this procedure has been lagging for decades although it is currently gaining momentum based on positive randomised data regarding prevention of recurrent ischaemic events4. Absorbable devices have been clinically tested in order to curb the reticence to implant a permanent device. Poor closure rates, higher thrombogenicity, and the fear of absorbable fabric chunks breaking off and embolising during resorption have put paid to the respective ambitions5-7. Likewise, applying a catheter-based stitch (HeartStitch® device; Sutura [now HeartStitch Inc., Fountain Valley, CA, USA]) or clip (Intersept by Cardica) to leave as little material behind as possible has been tried for over ten years. Used clinically very sporadically and by few operators, the technique did not produce satisfactory results. Therefore, there are no publications about the results. This is hardly surprising, as it is highly unlikely that a flimsy slit valve with a typical width of 1-2 cm can be closed by a single stitch that cannot even be centred (Figure 1). Likewise, surgeons had learned that a single stitch, in their case well centred, or even several interrupted sutures resulted in a high rate of residual shunts when they closed PFOs incidentally and rather cursorily during open heart operations. To be competitive with the fairly good closure rates of PFO closure devices, they reverted to uninterrupted sutures.
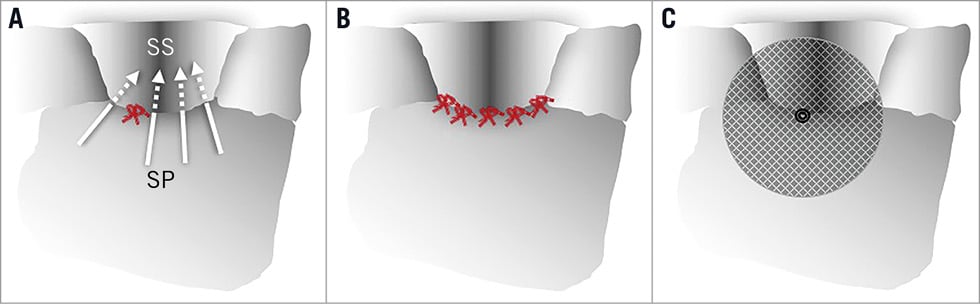
Figure 1. Depiction of various techniques to close the patent foramen ovale (PFO), viewed from the right atrium. A) A single and uncentred stitch leaves plenty of room for residual shunts (arrows). B) Five or more well placed stitches may do the job. C) AMPLATZER PFO occluder with a good potential for complete closure. SP: septum primum; SS: septum secundum
In the current issue of EuroIntervention, Gaspardone et al report a sizeable series of 200 patients treated with a modification of the failed HeartStitch device which is now called NobleStitch™ (HeartStitch Inc.) referring to the inventor, co-owner, and one of the authors8.
The modified device features two separate wire loops (rather than just one, as its predecessor had). Nonetheless, the two loops create only a single stitch. The rim of the usually flimsy septum primum is now intentionally folded back by the stitch in a sort of knuckle. According to the authors, this improves the closure rate, providing some kind of a skirt against a right-to-left shunt not unlike the principle used against paravalvular leaks in one of the valves for transcatheter aortic valve replacement.
Of 200 allegedly unselected patients requiring PFO closure, 192 were attempted and 186 (84%) had a technically successful procedure. Not quite as good as device closure which has 100% technical success if the PFO is passable, this success rate is still unexpectedly high. Intuitively, I would surmise that only PFOs with a minimal overlap of 5 mm are approachable. The required overlap may even be larger because of the attempted folding back of the rim of the septum primum. That might already disqualify about half of the PFOs and, with them, all the dangerous ones with a huge gap or a massive atrial septal aneurysm. Even in the remainder of patients, I would expect some residual shunt in the majority of cases according to what is shown in Figure 1. Surprisingly, the authors report a 75% closure rate at follow-up. We have to keep in mind that, when using transthoracic instead of transoesophageal echocardiography to check for residual shunt, as they did, 10%-30% of small shunts are probably missed, depending on how rigorously and sustained the Valsalva manoeuvre is practised with either method.
The authors rightfully mention some strengths of their technique, such as little overall material left inside. There is even less material (they incorrectly state none) in contact with the left atrial blood and there is no risk of erosion of a free wall during follow-up. They also anticipate a smaller risk of atrial fibrillation which is the only unavoidable downside of device closure, save the exquisitely rare erosion of a free atrial wall mentioned above9. Alluding to what they call a significant complication risk of device closure, the authors advocate their technique over and above the mere psychological benefit of providing the desired prevention without an implant. However, thrombus on the single stitch had been seen in earlier studies at least as frequently as thrombus on a device. Vascular complications, which in fact should not exist at all with venous punctures, will be found to be more common with the 14 Fr rather than 8-10 Fr sheath required. Clinically significant atrial fibrillation may or may not be less frequent than with device closure, with which it has to be expected in at worst 1% of patients. There is really not much trade-off that this technique offers to compensate for the poor closure rate. In three of the patients with significant right-to-left shunt at follow-up, the authors found a new atrial septal defect. According to them, these defects had been overlooked in the pre-procedure and in the intra-procedure transoesophageal echocardiograms. According to me, these defects represent suture tears of the septum primum, which is constantly in motion in some patients. This problem is known from surgical PFO closure but is hardly a problem with device closure. Modifying the NobleStitch technique to apply at least five stitches in a fan-like controlled fashion (Figure 1) would help but render the already complex and time-consuming procedure even more intricate.
I will continue to advise my patients striving for a deviceless PFO closure that for them, unfortunately, a stitch in time does not save nine. Why not consider the device as an internal tattoo or piercing, and simply ignore it and get on with life.
Conflict of interest statement
The author declares receiving speaker fees from Abbott.

