Abstract
Aims: Although routinely used, limited data are available regarding the long-term outcome after patent foramen ovale (PFO) closure using the HELEX® Occluder system. The aim of this study was therefore the examination of the acute and long-term outcome after transcatheter PFO closure using this system.
Methods and results: All (n=407) patients included had undergone PFO closure with the HELEX® Occluder system for secondary prevention of stroke, transient ischaemic attack (TIA) or peripheral embolism at a single centre. Primary endpoints were residual shunts at six or 12 months (assessed by transoesophageal echocardiography) and the number of neurological and other adverse events during follow-up. Device implantation was successful in 99% of patients. Complete closure at six months was achieved in 81%. During follow-up of 1,695 patient-years, 10 neurologic events occurred (four TIA, six strokes). The annual incidence of stroke was 1.2%. Other adverse events were wire frame fractures requiring no further intervention in five (1%), device-associated thrombus formation in one (0.25%), and paroxysmal atrial fibrillation in nine patients (2%).
Conclusions: PFO closure using the HELEX® Occluder system is feasible and safe. Complications and adverse events during long-term follow-up are rare. The safety profile and efficacy in prevention of recurrent events compare well to that reported with other closure devices.
Introduction
A patent foramen ovale (PFO) is an interatrial communication found in 17-20% of adult hearts1-3. Several studies have shown that the presence of a PFO in patients with a stroke in the absence of other known causes may increase the risk of recurrent events4,5. It is frequently the only suspect and therefore considered to be a common cause of stroke especially in young adults. In addition, an association between the presence of a PFO and migraine has been reported6-10.
The HELEX® Occluder system (W.L. Gore and Associates, Flagstaff, AZ, USA) is an umbrella device designed for atrial septal defect and PFO closure. The device has been available and has been in clinical use for many years. However, published data, particularly of long-term results, are limited11,12. The aim of our study was the evaluation of safety and efficacy, including long-term follow-up, in a large cohort of patients treated with the HELEX Occluder system.
Methods
We prospectively followed at our centre all consecutive patients who underwent percutaneous PFO closure using the HELEX® Occluder system. Selection of the HELEX Occluder system for PFO closure depended on operator preference and availability of devices. The presence of a PFO was verified by transoesophageal echocardiography (TEE). A TEE was performed at one and six months to assess whether there was residual shunting. Agitated saline was injected via peripheral venous access at rest and during Valsalva manoeuvre to assess the presence and magnitude of right-to-left shunting if any. One to five bubbles in the left atrium were considered a small shunt, 6-20 intermediate and more than 20 a large shunt13.
An atrial septal aneurysm was defined as an excursion of the septum of more than 10 mm beyond the midline14. The design of the HELEX Occluder and the implantation technique have been described before11,12. The implantation was performed under TEE and fluoroscopic control. The HELEX Occluder is available in the following sizes: 15 mm, 20 mm, 25 mm, 30 mm and 35 mm. Balloon sizing was performed in all patients. The device size was chosen such that the Occluder to defect size (determined by balloon sizing) ratio was approximately 2:1. Hence, the HELEX device was generally not used for defect sizes larger than 18 mm.
The original occluder system consisted of an outer control catheter and an inner guiding catheter, and was inserted via a 9 Fr sheath. The occluder was deployed and locked using a central mandrel. The modified delivery system included a proximal handle split into three parts: a mandrel with Luer lock, a control catheter attached to the retrieval cord and a delivery catheter. This allowed implantation using a guiding catheter, which was then introduced via a 10 Fr or 13 Fr sheath depending on whether or not a 0.035 inch guidewire was used as a rail for the delivery catheter. The modified system reduces the risk of a “missed eyelet” (failure to catch the eyelet of the occluder with the right atrial hook). Of note, in case of a missed eyelet, the occluder could still be retrieved using the retrieval cord and another occluder could be implanted.
All patients received aspirin 100 mg daily and clopidogrel 75 mg daily for at least six months after the intervention. We recommended endocarditis prophylaxis for six months.
Follow-up was performed at one and six months including patient history, 12-lead electrocardiogram (ECG) and TEE. For patients who underwent follow-up visits and examination by the referring physician, we obtained the follow-up visit records. At 12-month follow-up, transthoracic echocardiography was performed instead of TEE. Thereafter, follow-up was performed by patient questionnaires sent to patients once a year. When a questionnaire was not returned, the patient was called in person and interviewed for events.
If significant residual shunting was present at 12-month follow-up, implantation of a second device was considered. The potential benefits and risks of a second intervention/device versus continuation of medical therapy were discussed with the patients in detail and the decision left to the discretion of the patient. If a patient did not wish to proceed with implantation of a second device, only continuation of aspirin was recommended.
Statistical analysis
Results for continuous variables are reported with mean and standard deviation, and results for discrete data as medians and interquartile range (IQR). Calculations were made using the chi-square test, the Fisher’s exact test, Mann-Whitney U test or Kruskall-Wallis test, as appropriate. A p-value of <0.05 was considered significant. Event rates were calculated according to the Kaplan-Meier method. All statistical analysis was performed using SPSS PC (version 15.0; SPSS Inc, Chicago, IL, USA).
Results
Between 1999 and 2008, 407 patients were treated with the HELEX® Occluder system. Ages ranged from 19-82 years and mean patient age was 50 years (52% male) (Table 1).
Reasons for referral for PFO closure were TIA in 48%, stroke in 40%, decompression sickness in 2% and peripheral embolism in 3%. One of the patients had already had a surgical atrial septal defect (ASD) closure and a PFO was subsequently diagnosed. Concomitant diseases and cardiac risk factors were arterial hypertension in 133 (33%) and diabetes in 21 patients (5%) (Table 2). Six patients were found to have atrial fibrillation, which was paroxysmal in five. Thirty-one patients (8%) had reported a history of deep venous thrombosis (Table 1).
The stretched PFO diameter ranged between two to 20 mm (mean: 9±3 mm). In eight patients more than one defect was present and 138 had an atrial septal aneurysm. The 15 mm, 20 mm, 25 mm, 30 mm and 35 mm devices were used in 150, 165, 71, 18 and 3 patients, respectively. The original delivery system was used at our centre from December 1999 until October 2005 (322 patients). Subsequently, 85 patients were treated with the modified system. Significantly more patients treated with the original system had experienced a stroke prior to PFO closure, and had larger defects and atrial septal aneurysms compared with those treated with the modified system (51% vs. 36%, p=0.02; 9 vs. 8 mm, p=0.02; 41% vs. 26%, p=0.01). No statistically significant differences were found for the other demographical characteristics.
Periprocedural results
Four hundred and four of 407 attempted interventions were successful. The three remaining patients received a different occluder. In one patient, an additional ASD was discovered during the intervention and an Amplatzer® Occluder (St. Jude Medical, St. Paul, MN, USA) was implanted instead of the HELEX® Occluder. The second patient had a large atrial septal aneurysm. After two futile attempts a CardioSEAL® STARFlex™ (NMT Medical, Boston, MA, USA) was implanted successfully. In the third patient the occluder embolised, was successfully captured and removed using a snare and an Amplatzer Occluder was implanted. A very small anterior rim probably contributed to this complication.
The mean occluder diameter was 20 mm (SD 4 mm). Occluder to defect ratio was 2.15:1 and a 9 Fr or 10 Fr sheath was used in 350 (86%) of the interventions.
There were eleven cases of a missed eyelet. In addition, in one case the catheter could not be separated from the occluder causing the retrieval cord to fracture; however, the occluder could be retrieved. In another patient the mandrel disconnected before implantation of the occluder, and in two more patients the position of the occluder was assessed as inadequate. The first occluders were removed in all of these patients and another HELEX Occluder implanted during the same procedure.
Periprocedural device embolisation occurred in three patients, two immediately after deployment and one within 24 hours. The two devices that embolised during the procedure were retrieved from the aorta and pulmonary artery, respectively. Both were retrieved using a snare and both defects were closed during the same intervention. In the third patient, the HELEX Occluder had embolised into the aortic bifurcation. This was discovered during routine echocardiography one day after the intervention. The occluder was captured and another HELEX Occluder was implanted using the same procedure. The patient was released the following day without further sequelae.
One patient experienced a TIA 30 minutes after the procedure. The symptoms resolved spontaneously without further sequelae.
One patient developed a pericardial effusion post-interventionally and 300 ml of blood was aspirated during pericardiocentesis. The intervention was technically challenging requiring several attempts until the PFO could be crossed.
CLOSURE RATE
Mean follow-up time was 34+/-23 months. We analysed 1,658 patient-years. Four patients were lost to follow-up (0.9%). Six-month TEE follow-up was performed in 76%. In an additional 6%, a TEE was performed at a later follow-up. Hence, long-term TEE follow-up was available in 82% of the patients.
A residual shunt was present in 100 patients (24%) at one month and 58 patients (19%) at six months. Ten percent (n=6) of patients with residual shunt at six months had an intermediate or large shunt. In an additional 16 patients who had a residual shunt at six months, closure occurred only at long-term follow-up beyond six months. Therefore, at final follow-up, the closure rate was 87%.
Of note, patients were included in the closure rate calculation only until the second device was implanted. Closures that occurred after implantation of a second device were not included in our closure rate calculation.
LONG-TERM FOLLOW-UP
STROKE, TRANSIENT ISCHAEMIC ATTACKS AND PERIPHERAL EMBOLIC EVENTS
Two strokes occurred in the first year and four thereafter. In only one of these patients was residual shunting present. Four patients experienced a TIA, in two of whom a residual shunt was present. One patient had amaurosis fugax three months after the procedure. This patient did not have a residual shunt.
The annual risk for stroke after three years of follow-up was 1.2%. The Kaplan-Meier curve in Figure 1 demonstrates freedom from embolic events. At one-, two-, three- and four-year follow-up 99%, 98%, 96%, and 95% of the patients were free from neurologic events, respectively. The number of patients at risk was 356 at one-year, 308 at two-year, 250 at three-year and 191 at four-year follow-up.
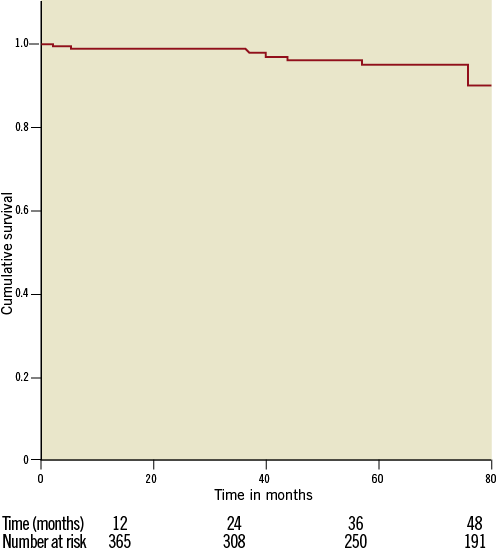
Figure 1. Survival free of neurological events
The adverse event rate for paradoxical embolism among patients with an atrial septal aneurysm was 1% during the first year, 0.6% during the second, 0% during the third and 1% during the fourth year of follow-up. There was no significant difference in event rates between this group and patients without atrial septal aneurysm (7 vs. 7; p=0.686).
ATRIAL FIBRILLATION
Nine patients (2%) were diagnosed with new-onset atrial fibrillation, seven of them during the first year. Two had only one short episode, three more converted to sinus rhythm spontaneously, one patient converted after medical treatment and one after electrical cardioversion.
DEVICE-ASSOCIATED THROMBUS
In one patient a thrombus was discovered on the occluder during a routine six-month follow-up (Figure 2). The patient had been taking aspirin at the time of the event, which was continued three further months at which time a follow-up visit revealed thrombus resolution. This case remained the only thrombus on a HELEX Occluder.
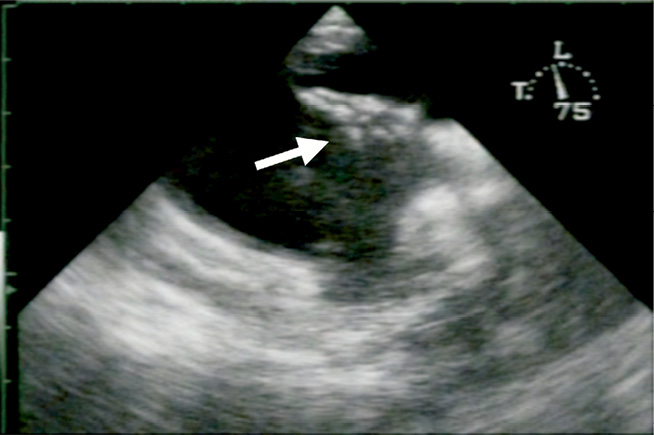
Figure 2. Thrombus on the right atrial side of a HELEX® Occluder. A thrombus (white arrow) can be seen on the right side of the HELEX Occluder.
LATE DEVICE EMBOLISATION
In one patient, at routine six-month follow-up, the occluder was discovered to have embolised into the aortic bifurcation. Transcatheter retrieval using a snare was unsuccessful probably due to tissue overgrowth. The position had caused a transaortic pressure gradient of 55 mmHG that was reduced to 5 mmHG by partial occluder detachment. In another patient embolisation to the aortic bifurcation was discovered at routine one-month follow-up. It was retrieved successfully percutaneously and an Amplatzer Occluder was implanted.
MISCELLANEOUS EVENTS
A fracture of the wire frame seen on fluoroscopy at six-month follow-up was reported in five patients, none of whom required further intervention.
One patient died three months after implantation due to pneumonia. No causal relationship to the intervention could be established.
In addition, the following events occurred during follow-up: one heart transplant at 28 months due to heart failure, one surgical PFO closure at 17 months due to a persistent shunt, one case of recurrent supraventricular tachycardia at 39 months, one death caused by subarachnoid haemorrhage at 13 months (unrelated to the PFO closure or device), and two more deaths at three and six years due to malignancy.
COMPARISON BETWEEN RESULTS OF THE ORIGINAL AND THE MODIFIED VERSION OF THE DELIVERY SYSTEM
In five patients treated with the original version of the HELEX® Occluder system a device wire fracture was discovered at follow-up. No such complication was seen in the group with the modified occluder. Furthermore, at one-year follow-up, 3% of the patients who received the original version of the occluder developed new atrial fibrillation, while no new onset atrial fibrillation was discovered in the group with the modified occluder.
In 11 patients (3.4%) treated with the original occluder system a missed eyelet syndrome occurred. This problem was solved with the modification of the occluder and was no longer seen with the more recent version. All cases of embolisation of the occluder happened with the original occluder system.
There were no differences in closure rates or recurrent neurologic event rates between the modified and the original version of the HELEX Occluder system (84% vs. 84%, p=1.0; 3% vs. 3%, p=1.0).
Discussion
We report long-term outcomes in a large cohort of patients who underwent PFO closure for secondary prevention of thromboembolic events using the HELEX® Occluder system. Several findings are important.
First, PFO closure with the HELEX Occluder system is accompanied by a high complete closure rate at long-term follow-up (87%). Ponnuthurai et al15 reported a three-month closure rate of 95.6% after PFO closure with the HELEX device in 69 patients. However, the closure rate may have been overestimated because assessment for residual shunting at follow-up was performed with transthoracic echocardiography, the sensitivity of which may be inferior to TEE for shunt detection. Sorensen et al16 reported a procedural success rate of 93% defined as Spencer grade ≤4 shunting at three-month transcranial Doppler examination with the Valsalva manoeuvre. Comparison with these data, however, are limited by the different imaging modes for the detection of residual shunts and by an arbitrary determination of what shunt magnitude by transcranial Doppler should be considered significant. Our closure rates with the HELEX device are similar to those observed with other available closure devices. Braun et al reported a closure rate using the Amplatzer PFO Occluder of 97% after twelve months and Wahl et al a closure rate of 79% using several different occluder systems17,18. Donti et al achieved a closure rate of 64% using the Premere Occluder system (St. Jude Medical, St. Paul, MN, USA)19. The fact that complete closure rates increase with time suggests that, similar to other devices, after HELEX device implantation, tissue overgrowth promotes complete closure.
Second, few adverse events, including neurological events, device associated thrombi, and wire fractures occurred. The annual stroke rate of 1.2% in our cohort at three years is at least equivalent to or lower than that reported with other devices. Wahl et al21 demonstrated a stroke, TIA and peripheral embolic event rate of 6.1% after one year, in patients treated with several different devices. In an analysis of 21 studies using closure devices for PFO closure, Staubach et al reported a recurrent event rate of 5.8% after up to 47 months of follow-up22. An annual event rate of 1.8% during a mean follow-up period of about two years was described using the Amplatzer PFO Occluder and the CardioSEAL Occluder23.
Third, the incidence of device-associated thrombi, a potentially devastating event, was very low. After completion of follow-up, the incidence of thrombus formation was 0.25%. This compares favourably to the reported device-associated thrombus rates of other closure systems. Krumsdorf et al analysed the incidence of thrombus formation after transcatheter closure of interatrial shunts with a variety of closure devices in a large patient population24. Thrombi were found in 2.5% of 593 patients after PFO closure during a mean follow-up time of 36 months. Fischer et al reported 5/154 thrombi on the STARFlex Occluder after six months of follow-up25. Wahl et al reported thrombus formation in 0.7% of 525 patients after PFO closure. The low incidence of thrombus formation with the HELEX Occluder could be related to the ePTFE (expanded polytetrafluoroethylene) membrane covering the wire frame of the device perhaps preventing thrombus formation.
Fourth, a higher event rate of stroke and TIA after PFO closure in patients with both a PFO and atrial septal aneurysm has recently been described20,26,27 but was not evident in our cohort. This could be related to the flexibility of the two device umbrellas, well-suited to a hypermobile interatrial septum.
Device embolisation occurred in 1% of patients (three early and two late embolisations). Numerically, this is higher compared to other devices in the only currently available study that compares the HELEX device to others in a randomised fashion28. Definitive statements regarding the likelihood of this complication compared to other devices cannot be made. Possible explanations for a potentially higher embolisation rate are the flexible structure of the device and, perhaps, more frequent choice of this device in patients with atrial septal aneurysms to which it appears to conform well but in whom device embolisation may be more likely. Alternatively, better handling of the modified device may be associated with a smaller risk of embolisation as all device embolisations in our study occurred with the original device.
Finally, modifications made to the occluder system in the course of clinical application have improved the procedural success. The missed eyelet syndrome no longer occurred with the modified device. Though more patients developed atrial fibrillation with the original occluder system, a definitive statement comparing both device versions cannot be made due to the small number of events.
Over the course of the years the overall complication rates were reduced as well, which may be partly related to system modifications and a learning curve. Nevertheless, complete absence of device embolisation suggests better device handling and more secure positioning.
Limitations
The most important limitation of our study is the restriction of data to a single centre. Given the non-randomised unblinded nature of our study, the results are subject to selection and observer bias. Therefore, definitive statements regarding efficacy when compared to medical therapy or other devices cannot be made.
A number of different devices for PFO closure are used at our centre. The device choice was left to the discretion of the interventionalist performing PFO closure. Therefore, a selection bias is an important limitation and more or less favourable results may have been obtained in an unselected patient population. Furthermore, pre- or post-procedural echocardiographic images were not evaluated in an independent corelab allowing observer bias. Finally, clinical follow-up was not performed by neurologists who would be best qualified to detect recurrent neurological events. Thus, it is possible that more subtle neurological events were not detected.
Conclusion
We conclude that percutaneous interventional patent foramen ovale closure using the HELEX® Occluder system is feasible and safe. Long-term recurrent event rates are at least as low as those described with other devices. The device design may prevent the formation of device-associated thrombi. Modifications on the occluder system allowed easier handling and reduced periprocedural complications.
Conflict of interest statement
H. Sievert has received study honorariums, travel expenses, consulting fees from Abbott, Access Closure, AGA, Angiomed, Arstasis, Atritech, Atrium, Avinger, Bard, Boston Scientific, Bridgepoint, Cardiac Dimensions, CardioKinetix, CardioMEMS, Coherex, Contego, CSI, EndoCross, EndoTex, Epitek, Evalve, ev3, FlowCardia, Gore, Guidant, Guided Delivery Systems, Inc., InSeal Medical, Lumen Biomedical, HLT, Kensey Nash, Kyoto Medical, Lifetech, Lutonix, Medinol, Medtronic, NDC, NMT, OAS, Occlutech, Osprey, Ovalis, Pathway, PendraCare, Percardia, pfm Medical, Rox Medical, Sadra, Sorin, Spectranetics, SquareOne, Trireme, Trivascular, Velocimed and Veryan. H. Sievert has stock options in Cardiokinetix, Access Closure, Velocimed, Lumen Biomedical, Coherex and SMT. The other authors have no conflicts of interest to declare.

