Abstract
Aims: Closure of patent foramen ovale (PFO) is non-inferior to medical treatment for patients with cryptogenic stroke. Results in randomised trials might be based on the different types of used occluders. We determined residual shunting with serial contrast transoesophageal echocardiography (cTEE) and evaluated rates of recurrent cerebrovascular events in a long-term follow-up.
Methods and results: cTEE was repeated three and 12 months after PFO closure using AMPLATZER (n=109), BioSTAR (n=68), Cardia (n=104) or Premere (n=54) occluders. Closure was demonstrated in 91.6% and 95.9% of patients after three and 12 months. Closure rates were not different among groups (p=0.58; p=0.94). The PFO diameter was a risk factor for residual shunting (p=0.02), but not the prevalence of an atrial septal aneurysm (ASA). During follow-up, including 1,815 patient-years (PY), eight patients suffered a stroke (0.44/100 PY) and seven patients a transient ischaemic attack (0.39/100 PY). Rates of recurrent cerebrovascular events were similar among the four groups.
Conclusions: Closure at three or 12 months (as measured by cTEE) and rates of recurrent cerebrovascular events were similar among occluder groups. PFO diameter was a risk factor for residual shunting, but not the presence of ASA. The rate of recurrent cerebral ischaemic events was low.
Introduction
Patent foramen ovale (PFO) has been linked to several disorders such as cryptogenic embolic events, migraine, decompression illness and platypnoea orthodeoxia. The cerebral circulation is mainly involved in patients with cryptogenic embolic events based on presumed paradoxical embolism, but other vessel territories such as coronary arteries are also affected1. PFO closure in those patients has been discussed2,3. Several meta-analyses4,5 and a propensity score-matched study6 showed a lower rate of recurrent stroke after percutaneous transcatheter closure of PFO compared to medical treatment in patients with cryptogenic embolic events. Three randomised trials showed non-inferiority of transcatheter PFO closure compared with medical treatment alone for the prevention of recurrent stroke or transient ischaemic attack (TIA) in patients with cryptogenic cerebral events and PFO. In the RESPECT and PC trials7,8, there was a clear trend towards a lower recurrence rate after AMPLATZER™ device (St. Jude Medical, St. Paul, MN, USA) implantation, compared with medical treatment alone. In contrast, in the CLOSURE I trial9 device implantation showed similar results compared with medical therapy. These results were obtained with the STARFlex occluder, (NMT Medical, Boston, MA, USA) a closure device with a relatively low efficacy regarding closure, as reported in this trial, and an increased risk for thrombus formation compared to other devices10-14. Differences between these studies may be based on a different efficacy regarding closure of the PFO after device implantation. The acquisition of differences in the clinical outcome of these two treatment strategies requires long-term follow-up. Contrast transoesophageal echocardiography (cTEE) is the optimal method to discriminate the efficacy of PFO closure among different devices.
We evaluated in a large, prospective, observational registry the efficacy of different PFO devices regarding complete closure assessed by serial cTEE, and the rate of recurrent cerebrovascular events (RCE) in a clinical long-term follow-up.
Methods
Patients (N=335) with PFO presenting with cryptogenic ischaemic events (stroke or TIA) were enrolled in this prospective, observational registry after successful device implantation for PFO closure and TEE at three-month follow-up. Stroke or TIA was diagnosed by neurologists using cranial computed tomography (cCT) or cranial magnetic resonance imaging (cMRI) in combination with the clinical presentation of the patient. Cryptogenic stroke was defined as brain infarction that was not attributable to a source of definite cardioembolism, large artery atherosclerosis or small artery disease, despite extensive vascular, cardiac and serologic evaluation according to the TOAST classification15. Cryptogenic TIA was defined as a transient episode of neurologic dysfunction without documentation of ischaemia in cerebral imaging.
All patients underwent a diagnostic evaluation with routine laboratory testing, carotid duplex ultrasonography and transcranial doppler ultrasonography (TCD), transthoracic and transoesophageal echocardiography (TTE and TEE). All patients demonstrated moderate or severe right-to-left shunting in cTEE. Sinus rhythm was documented for all patients in 12-lead and Holter electrocardiography (ECG). No patient had a history of atrial tachycardia, atrial flutter, or atrial fibrillation. Patients in need of anticoagulation therapy, due to other disorders, were excluded. cTEE was performed with a multiplane, phased array 4- to 7-MHz transoesophageal echocardiography probe on an ATL HDI 5000 CV (Philips Medical Systems, Best, The Netherlands) or iE33 xMATRIX (Philips Deutschland GmbH, Hamburg, Germany). Valsalva-induced right-to-left shunt was graded according to the number of bubbles crossing the interatrial septum after the injection of 10 mL of agitated hydroxyethyl starch solution via a cubital vein. No shunt was defined as zero bubbles, trace shunt one to nine bubbles, moderate shunt 10 to 19 bubbles, and severe shunt ≥20 bubbles. Bubble testing was performed three times with the proper Valsalva manoeuvre as described elsewhere16.Size and morphology of the PFO were assessed by cTEE. PFO diameter was measured in the short axis as the maximum opening of the communicating channel. Closure was defined as no or trace residual shunt assessed by cTEE. An atrial septal aneurysm (ASA) was determined as an excursion of the atrial septum >10 mm17. Patients were scheduled for cTEE at three and 12 months after device implantation. Patients were followed up for RCE by assessing their clinical history at scheduled outpatient controls or through telephone contacts. At each contact the same questionnaire (stroke, TIA, bleeding, arrhythmia, endocarditis, device dislocation and other complications) was used. For reported recurrent stroke or TIA, medical reports, including imaging procedures, were collected from the neurologists assessing the recurrent event.
Devices
Four PFO devices were studied (Figure 1). PFO devices were implanted in the catheterisation laboratory as described elsewhere18,19. The devices were delivered through an appropriate sheath depending on the size of the selected occluder. Deployment and position of the device were controlled by fluoroscopy and by periprocedural TEE.
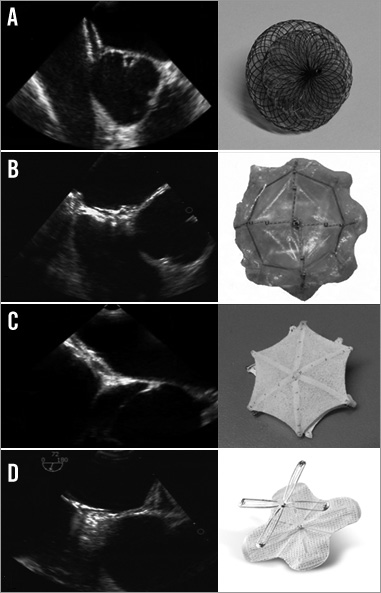
Figure 1. TEE images and pictures of the different occluder types. TEE short-axis views of: A) AMPLATZER PFO, B) BioSTAR, C) Cardia PFO, D) Premere.
The BioSTAR septal repair implant (NMT Medical) was an innovative bioabsorbable device. It consisted of a purified porcine intestinal collagen layer matrix mounted on an MP35N STARFlex (NMT Medical) “double-umbrella” framework, coated with a heparin benzalkonium chloride complex20. Nitinol microsprings between the right and the left atrial disc allowed a self-centring of the device. The collagen matrix is absorbed and entirely replaced by host tissue over a period of approximately 24 months. The AMPLATZER PFO occluder (St. Jude Medical, St Paul, MN, USA) is a self-expandable, double disc device made from a nitinol wire mesh. The two flat discs, which are linked by a connecting waist, contain thin polyester fabric. In this series the AMPLATZER PFO occluder, the AMPLATZER septal occluder and the AMPLATZER cribriform occluder were implanted. The Cardia PFO occluder (Cardia Inc., Eagan, MN, USA) is made of two polyvinyl alcohol sails, mounted on six nitinol wires for each sail, connected by a centre post. In this series patients received generation I-III Cardia PFO or Cardia ASD occluders. The Premere™ PFO Closure system (St. Jude Medical, St Paul, MN, USA) included thin, flexible, nitinol anchors. An adjustable tether connected the left and right anchors, allowing an adaptation to different tunnel lengths. The AMPLATZER and Cardia devices were used for the whole study period. There were two consecutive series with the Premere device and the BioSTAR in which the AMPLATZER and Cardia devices served as back-up devices at the discretion of the operator in a more complex anatomy (e.g., very large PFO or septal aneurysm).
Patients received a combined antiplatelet therapy for three to six months with acetylsalicylic acid 100 mg per day and clopidogrel 75 mg per day. Patients were scheduled for follow-up cTEE three and 12 months after device implantation, according to institutional routine practice. In case of persistent moderate or severe shunting we did not perform another device implantation.
STATISTICAL ANALYSIS
Categorical parameters are presented as counts and percentages and were compared by Pearson’s chi-square test. Continuous variables are presented as mean±SD, and were compared using the Kruskal-Wallis non-parametrical ANOVA. The primary outcome measure was closure on cTEE, defined as no or trace residual shunt after a three-month follow-up. A multiple regression analysis including PFO diameter, presence of ASA and occluder type was performed to analyse their predictive value regarding effective closure after three and 12 months. The log-rank test was used to calculate the influence of occluder type on event-free survival.
A p-value <0.05 was considered to be statistically significant. Statistical analysis was performed using STATISTICA, release 7.1 (StatSoft, Inc., Tulsa, OK, USA).
Results
Patients received AMPLATZER devices (n=109), BioSTAR occluder (n=68), Cardia occluder (n=104) or Premere PFO closure system (n=54). The clinical indication for PFO closure was cryptogenic stroke (69.6%), TIA (23.3%), peripheral arterial embolism (3.9%) or myocardial infarction (3.3%). One patient developed periprocedural pericardial effusion (0.3%), which was subsequently successfully drained by pericardiocentesis. There was no device embolisation.
Baseline parameters (Table 1) did not differ. PFO characteristics are shown in Table 2. PFO diameter, presence of ASA and pre-procedural shunting did differ between groups (Table 2). PFO diameter was largest in the AMPLATZER and Cardia groups, presence of ASA was most frequent in the BioSTAR and Premere groups, and severe shunting was most often seen in the AMPLATZER and Cardia groups.
cTEE was performed on all patients after three months and was repeated on 271 patients (80.9%) after 12 months. The primary outcome measure, closure at three months, was present in 91.6% of patients and increased to 95.9% at 12-month cTEE. Closure rates at three-month (p=0.58) and 12-month cTEE (p=0.94) were statistically not different among the four devices (Figure 2, Figure 3). After twelve months, a cTEE was performed in 21 (75%) of 28 patients with residual moderate or severe shunt at three-month cTEE. Closure after 12 months was demonstrated in 61.9% (n=13/21) of these patients. The occurrence of closure betweenthree-month and 12-month cTEE did not differ statistically among the four groups (p=0.74).
Figure 2. Closure rates at three months by cTEE.
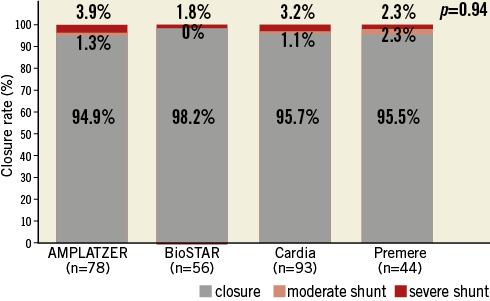
Figure 3. Closure rates at 12 months by cTEE.
Baseline shunt, index event for PFO closure, cardiovascular risk factors, gender and age were not different between patients with and without closure. In patients with closure within three months, the baseline PFO diameter was 12.5±4.1 mm compared with 14.5±4.5 mm in patients with moderate or severe residual shunt (p=0.056). In patients with documented closure at 12 months, the baseline PFO diameter was significantly smaller with 12.3±4.0 mm compared with 15.3±4.3 mm in patients with moderate or severe residual shunt (p=0.024). The PFO diameter in patients with documented closure between three and 12 months was smaller than in those without closure (12.2±3.3 mm versus 14.5±4.8 mm, p=0.85).
The presence of ASA did not influence closure rates at three and 12 months. Closure in patients with ASA was present in 90.8% at 3 months and 96.1% at 12 months, compared with 93.5% (p=0.40) and 95.6% (p=0.84) in patients without ASA, respectively.
Closure rates among our four devices did not differ at three and 12 months in patients with severe baseline shunt (p=0.23; p=0.8), in patients with a PFO diameter equal to or larger than median (13 mm) (p=0.17; p=0.24), and in patients with ASA (p=0.65; p=0.71).
In multivariable analysis there was a trend for the PFO diameter to be a risk factor for relevant residual shunting at three months (p=0.06). PFO diameter was a significant risk factor for relevant residual shunting at 12-month cTEE (p=0.02). Closure rates at three or 12 months were independent from occluder type (p=0.23; p=0.99) or the presence of ASA (p=0.43; p=0.71).
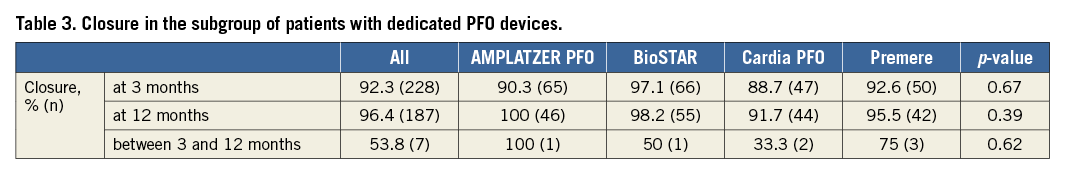
A subgroup analysis including patients with a dedicated PFO device (excluding ASD devices) showed no statistically significant difference in closure rate after three-month and 12-month cTEE (Table 3).
CLINICAL FOLLOW-UP
Clinical follow-up was available for 332 (99.1%) patients with a median of 5.1 years, range 9.3 months to 12.3 years. Follow-up included a total of 1,815 patient-years (PY). Median follow-up was longest in the Cardia group with nine years, followed by AMPLATZER with five years, Premere 4.6 years and the BioSTAR group with 3.3 years. During follow-up, eight patients suffered a stroke (0.44/100 PY) and seven patients (0.39/100 PY) a TIA. One haemorrhagic stroke was diagnosed 141 days after the implantation procedure, with the patient being on combined antiplatelet therapy. All other patients showed ischaemic RCE. No fatal stroke was observed. Four patients died of non-vascular causes.
Five patients in the Cardia group and three in the Premere group suffered a recurrent stroke. TIA was documented in three patients in the Cardia group, two patients in the AMPLATZER group, one patient in the Premere and one patient in the BioSTAR group. The rate of RCE/100 PY for the different occluders was significantly not different (p=0.72) with 0.9 for Cardia, 0.4 for AMPLATZER, 1.7 for Premere and 0.5 for BioSTAR.
The rate of RCE was not influenced by the presence of ASA before PFO closure (p=0.18). The rate of RCE/100 PY for the patient group with a PFO diameter above the median was 1.05 and for the patient group with a PFO diameter equal to or below the median 0.66 (p=0.40).
New onset atrial fibrillation was documented in three patients (4.4%) in the BioSTAR, two patients (2%) in the Amplatzer, one patient (1%) in the Cardia and one patient (1.9%) in the Premere group (p=0.81). None of these patients showed persistent episodes of atrial fibrillation or cerebrovascular events during follow-up.
Discussion
Previous studies comparing the effectiveness of different devices, using either transthoracic or transoesophageal contrast echocardiography, showed that closure rates may be different among device types11,13,21,22. Residual shunting after percutaneous PFO closure has been documented as a risk factor for recurrent paradoxical embolism23. We determined residual shunting in a large population with routine cTEE at three and 12 months and evaluated rates of recurrent stroke or TIA in a long-term follow-up.
In our population, closure rates did not differ statistically among the four device types. Furthermore, rates of RCE were similar among the four occluder groups. The pre-procedural diameter of PFO was a risk factor for residual shunting but not the presence of an ASA. We used cTEE for assessment of residual shunting since it is superior compared to cardiac magnetic resonance imaging18,24, and also superior to TTE, real-time three-dimensional TTE and transcranial Doppler because of a high sensitivity and a high negative predictive value25,26.
Thaman et al compared three devices after percutaneous closure in patients with large PFO (≥30 bubbles after Valsalva) using contrast transthoracic echocardiography (cTTE), finding a significantly lower rate of residual shunting for the AMPLATZER PFO compared to the HELEX occluder, (Gore Medical, Flagstaff, AZ, USA). The Premere device showed an intermediate residual shunt rate21. In a cohort of patients with atrial septal defect (ASD) or PFO and percutaneous implantation of the bioabsorbable BioSTAR device, closure was demonstrated in 54 of 56 patients (96%) using cTTE20. In another series with this bioabsorbable device, rates for relevant (moderate or severe) residual shunting were low after 12 months with 7.2% and remained similar after 24 months with 9.1% (p=0.37)27. We now present the largest experience with the bioabsorbable BioSTAR device. There were no differences in closure rates at three and 12 months compared with the AMPLATZER, Cardia and Premere PFO closure systems. However, we found high closure rates in the BioSTAR group even after three months, which might be explained by an optimal and early adherence of collagen to the atrial septum. Although BioSTAR is no longer available, this might be an important issue for new collagen-based, bioabsorbable devices in development, allowing a later simpler access to the left atrium.
Von Bardeleben et al13 found that not only the closure rates but also the closure time curves were dependent on occluder type comparing AMPLATZER PFO, STARFlex and HELEX occluders in 357 patients using cTEE. With large HELEX occluders there was a time delay of four months to achieve the same closure rates as the AMPLATZER and STARFlex. In addition, Taaffe et al found significant differences in complete closure rates 30 days after randomised implantation of AMPLATZER, CardioSEAL/STARFlex and HELEX occluders in 660 patients using cTEE11. After a follow-up period of five years, complete PFO closure was documented in 100%, 99.5% and 96.8%, respectively28. The overall effective closure rate was 91.6% at three months and increased to 95.9% at 12 months. These data and our results support performance of a cTEE control at 12 months after device implantation, after complete device endothelialisation. Furthermore, the rate of clinical events was low and comparable to the clinical results of the randomised PC trial8 and RESPECT study7, in which transcatheter implantation of an AMPLATZER PFO occluder was compared to best medical therapy. Within the device groups the occurrence of stroke/TIA was 3% (at a median follow-up of 4.1 years) in the PC trial. Numbers for the RESPECT study were 1.33% and 2.21% after one and five years of follow-up, respectively. In the per protocol and as-treated cohorts of the RESPECT trial there was a significant reduction of clinical endpoints with device implantation compared to medical therapy. In a pooled analysis of the RESPECT and PC trials, the stroke estimate became statistically significant (hazard ratio 0.44, 95% confidence interval: 0.20-0.95; p=0.04)29. Closure rates of the AMPLATZER PFO device at six-month cTEE were high with 95.9% in the PC trial and 93.5% in the RESPECT study. In contrast, effective closure with use of the STARFlex device was clearly lower (86%) in the CLOSURE I trial9, showing no relevant clinical benefit compared to medical therapy.
The initial presence of an ASA did not influence closure rates. This is in contrast to medical therapy, in which the presence of an ASA puts the patient at an increased risk for recurrent events in the long term30-32. In the RESPECT trial, the subgroup of patients with cryptogenic ischaemic events with PFO and an ASA did significantly benefit from AMPLATZER PFO device implantation compared with medical therapy alone7. The low rate of clinical events in our population with more than 60% ASA supports the implantation of PFO occluders for minimising the risk of recurrent events. According to our cTEE study, the main risk factor for residual shunting is the PFO size at baseline and not the presence of ASA or type of implanted device. With comparable closure rates for PFO devices, other factors such as a simple and fast procedure, new onset of atrial fibrillation, thrombus formation and smaller size of delivery sheath to avoid vascular complications become important for device selection. Documentation of new-onset atrial fibrillation in our population ranged between 1% and 4.4%, with not statistically significant higher rates with the BioSTAR device. The BioSTAR device has the same nitinol wire frame as the STARFlex device, which also demonstrated higher rates of new onset of atrial fibrillation7-9. However, whether new onset of atrial fibrillation is due to the implanted device, or could have been previously diagnosed with an insertable cardiac monitor33 or a 30-day event-triggered recorder34, remains unclear.
Limitations
This is an observational registry and not a randomised trial. Some devices were dedicated to a special PFO morphology (Premere device). On the other hand, the Cardia ASD and AMPLATZER ASD occluders always served as back-up devices for large PFO diameters. This may explain the differences in baseline data. Only early generations of Cardia devices were studied. Our results may not be translated to the newest generation of devices.
Conclusion
We were able to demonstrate in a large prospective registry including routine three-month and 12-month cTEE that closure rates after device implantation for PFO closure did not differ among the four occluder types studied. With the AMPLATZER PFO device there was a 100% closure rate after 12 months. The diameter of PFO prior to device implantation was a risk factor for residual shunting seen in cTEE but not the documentation of an ASA at baseline. The rate of recurrent cerebral ischaemic events was low and did not differ among the devices used.
| Impact on daily practice We demonstrate that, in patients with cryptogenic ischaemic events, the closure rate of PFO by dedicated devices is high, resulting in a low rate of recurrent cerebrovascular events. Results did not differ among the four occluders implanted. Furthermore, the rate of PFO closure increased within three to 12 months of follow-up. The final result after device implantation should be evaluated after 12 months. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

