
Abstract
Aims: The aim of this study was to evaluate local biological responses to the partially bioresorbable non-metal frame Carag bioresorbable septal occluder system in an experimental setting.
Methods and results: A Good Laboratory Practices (GLP) study was performed with implantation of the device into the interatrial septum of 24 German Landrace pigs with follow-up periods of 3, 5, 8 and 15 months (six animals in each group). One non-implant-related death occurred one month after implantation. Histology was obtained by sawing and grinding of the hard-resin embedded specimen after formalin fixation. All occlusion devices were found correctly positioned without any residual shunt at the end of the experiments. Complete endothelialisation could be confirmed histologically in all specimens independent of implantation period. There were only a few lymphocytic infiltrations locally related to the implant materials. Sporadic macrophages and foreign body giant cells were found adjacent to the textile fabric. Resorption of the biodegradable frame material was seen to proceed with implantation time.
Conclusions: This is the first report on histopathology of a septal defect occluder with a bioresorbable filament structure in vivo which is already in clinical use. Good biocompatibility was demonstrated with documentation of timely degradation and substitution of the polymer material by fibromuscular cells and extracellular matrix components.
Abbreviations
ASA: acetylsalicylic acid
ASD: atrial septal defect
CBSO: Carag bioresorbable septal occluder
ECG: electrocardiogram
GLP: good laboratory practice
PEEK: polyetheretherketone
PLGA: poly(lactic-co-glycolic acid)
Pt-Ir: platinum-iridium
Introduction
To date, all commercially available devices for interventional closure of cardiac septal defects are made of permanent materials. This results in the presence of a foreign body in the patient throughout life.
Clinical interest and information in the literature on interventional septal defect closure devices are generally focused on feasibility, safety and long-term clinical outcome. In recent years, biocompatibility has additionally emerged as a focus of interest1-5. In this context, chronically persisting inflammatory reactions have been described, consisting mainly of lymphocytic infiltrations and foreign body reaction locally related to foreign materials6. Furthermore, thrombus formation at the surface of atrial septal occluders has been seen even years after implantation, most likely related to incomplete endothelialisation7,8. Also, cardiac erosion is one of the fears in permanent occlusion devices. However, most importantly, a lifelong permanent implant is not needed since proliferating tissue components overgrow cardiovascular devices and overtake the function of the implant, therefore making it dispensable6,9,10. Thus, there is a reason and a clinical need for the development of bioresorbable implants in order to reduce possible long-term complications.
The aim of the present study was the evaluation and description of tissue reactions after implantation of a septal defect occluder using a bioresorbable framework that was tested in an animal model prior to introduction into clinical use.
Methods
DEVICE
The Carag bioresorbable septal occluder (CBSO; CARAG AG, Baar, Switzerland) is a self-centring device with two opposing foldable non-resorbable polyester covers, each of which is attached to a framework consisting of a poly(lactic-co-glycolic acid) (PLGA) monofilament, a well-described bioresorbable material (Figure 1). PLGA has been used in medical device applications for years, including cardiac applications such as coronary stents. The fabric is secured to the monofilaments by platinum-iridium (Pt-Ir) markers and non-absorbable surgical sutures composed of polypropylene. The entire framework comprises eight resorbable filaments. The implant is designed such that the distal cover sits against the left atrial septum, and the proximal cover sits against the right atrial septum. At each end of the filaments, there is a filament holder made of polyetheretherketone (PEEK), a non-resorbable polymer used in medical device applications as well. At the distal tip of the implant, a nut made of Phynox (a cobalt-chromium-nickel alloy) keeps the filaments in place and ensures visibility under X-ray. The occluder is delivered through a 12 Fr sheath over a 0.018 inch guidewire. The implant is loaded into a peel-away sheath, which serves to compress the implant to a small size prior to delivery to the heart. The delivery system consists of two coaxial control catheters that allow an independent control of the distal and the proximal end of the device. It is used to deploy and lock the implant by connecting both ends following re-expansion on the septum. Two sizes of CBSO were used: type S with an outer diameter of 26 mm to treat defects from 4 to 12 mm, and type M with an outer diameter of 28 mm to treat defects from 11 to 20 mm.
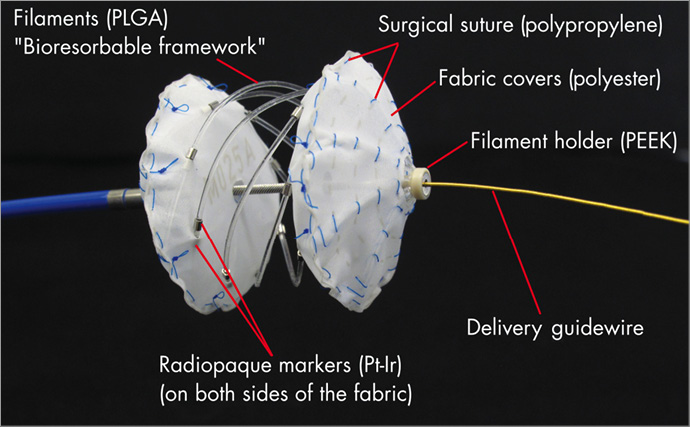
Figure 1. CBSO device. The CBSO device connected to the delivery catheter with the delivery guidewire after partial unfolding.
ANIMAL EXPERIMENTS
Twenty-four CBSO were implanted in growing healthy German Landrace pigs. Animals underwent endotracheal intubation and were mechanically ventilated with isofluorane and oxygen/room air. ECG, heart rate, respiratory rate, transcutaneous oxygen saturation, tidal volume and end-tidal CO2 were monitored throughout the procedure. An artificial defect in the atrial septum was created by transseptal puncture followed by dilatation with a 12 mm balloon in 12 animals, and a 16 mm balloon in another 12 animals. All animals received 5 mg acetylsalicylic acid (ASA) per kg daily from implantation to euthanasia.
All animals received adequate care in compliance with the “Animal Welfare Act” of May 1998, published in the German Federal Law Gazette I, page 1105. The study had been approved and supervised by the local competent authority.
At the end of the observation period, the animals were euthanised and a sternotomy was performed to remove the heart and lung package.
TISSUE PREPARATION
Immediately after explantation, the tissue block containing the implant was dissected free with a minimum of surrounding tissue. After briefly flushing with saline, the specimens were fixed in formalin (buffered 4%).
EMBEDDING, SECTIONING, AND HISTOLOGY
Prior to embedding, macroscopic evaluation and documentation was accomplished. After fixation, the tissue block with the device was embedded in hard-resin methyl methacrylate (Technovit® 9100; Kulzer GmbH, Wehrheim, Germany). Following hardening, the resin blocks were subsequently sectioned into slices using a diamond band saw (Diamant Saw 300; Walter Messner GmbH, Oststeinbek, Germany). These slices were ground down to 15-20 µm with a horizontal rotary grinder and polisher (Exakt - micro grinding systems; Walter Messner GmbH). Standard staining was performed with Richardson’s blue.
A four-stage grading system was applied for quantification of cellular inflammatory reactions. Therefore, different types of inflammatory cells were counted in high-power fields (magnification 400×) and subsequently averaged and graded as described by Cohen11.
STATEMENT OF RESPONSIBILITY
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Twelve type S devices were deployed in the 12 mm defects, and twelve type M devices were deployed in the 16 mm defects, all using standard techniques under control by fluoroscopy and intracardiac echocardiography. Colour Doppler echocardiography was used to assess potential residual shunting. All animals received ASA (5 mg/kg per day) during follow-up. Follow-up periods were 3, 5, 8, and 15 months for six animals (three type S, three type M) in each group.
No intraprocedural complications occurred during implantation. All devices were implanted into the interatrial septum defect without dislocation or residual shunts at the end of the implantation procedure. One animal had to be euthanised during follow-up one month after implantation due to bad health. Gross pathology was performed showing correct positioning of the devices and almost complete endothelialisation. After careful examination, the suspected cause for the clinical deterioration was an acute stress myopathy, for which German Landrace pigs have a genetic disposition. No evidence was found for an implant-related cause. The animal was replaced.
GROSS PATHOLOGY
All devices were macroscopically intact. No compromise of blood flow at the pulmonary or systemic veins or interference with the heart valves was seen in any of the animals. This had been documented concordantly prior to euthanasia by means of echocardiography.
Superficially, the explants appeared completely covered by a thin and smooth whitish tissue layer (Figure 2). No parts of the occlusion devices were found directly exposed to the blood stream. On careful examination, there was no evidence of superficial thrombus formation in any of the specimens evaluated for this study. This finding was independent of the time interval between implantation and explantation.
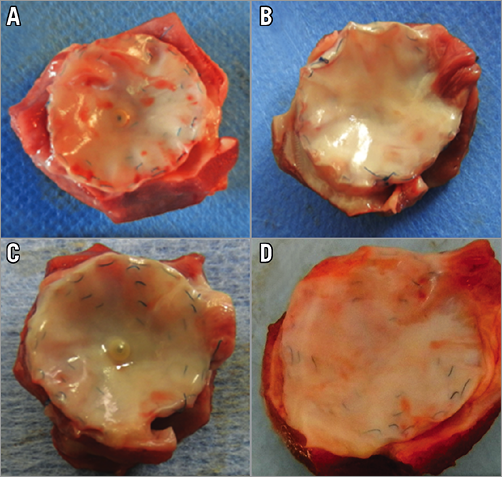
Figure 2. Macroscopy. Macroscopic aspect of explanted occlusion devices after an implantation time of 3 months (A), 5 months (B), 8 months (C), and 15 months (D) showing progressive overgrowth of the explants with fibrous tissue dependent on implantation time.
The configuration of the devices was evaluated histologically after production of ground sections through the central part of the specimen (Figure 3). A regular and even configuration of all devices was demonstrated, again independently of implantation time. The superficial tissue proliferation led to complete incorporation of the prominent parts of the central locking mechanism into the pseudointima without a demonstration of immoderate proliferation over time.
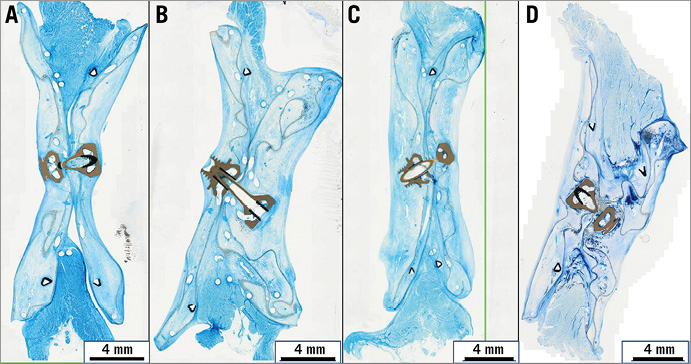
Figure 3. Configuration of devices. Representative histologic images for the four experimental groups of ground sections through the centre of the occlusion devices after an implantation time of 3 months (A), 5 months (B), 8 months (C), and 15 months (D). Richardson staining (blue staining of tissue components). The centrally located grey and black structures represent the filament holder and the locking mechanism.
BLOOD-IMPLANT INTERFACE
Complete endothelialisation was demonstrated in all four implantation interval groups (Figure 4). Even the edges of the polymer cover and prominent parts of the central locking mechanism were completely covered by a partially very thin tissue layer. In accordance with macroscopic findings, no superficial fibrin condensations or thrombus formation were found histologically.
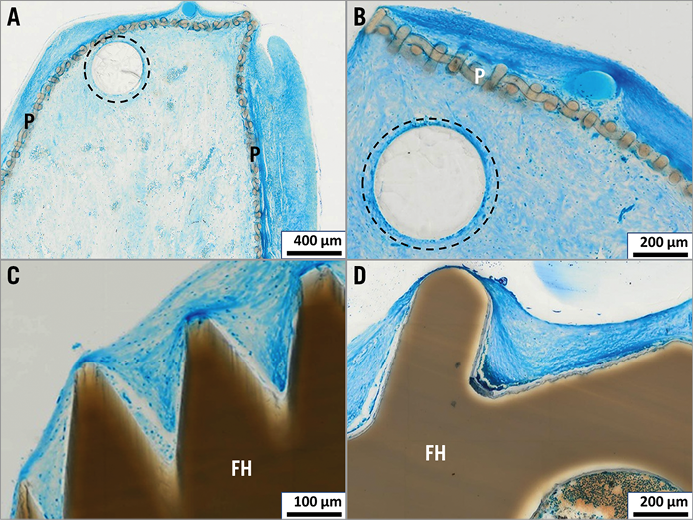
Figure 4. Endothelialisation. The outer part of the polyester cover (P) is superficially coated with a thin layer of tissue (panel A, implantation time 3 months; panel B, implantation time 5 months); prominent parts of the filament holder (FH) are likewise covered with tissue and superficially with a definable one-cell layer of endothelium (panel C, implantation time 5 months; panel D, implantation time 8 months). Bioresorbable PLGA monofilaments encircled by dotted line.
TISSUE ORGANISATION AND IMPLANT DEGRADATION
Cellular organisation of the tissue components within the occlusion devices was advanced and almost completed even in the shortest running implant group (three months). Completed cellular organisation was demonstrated for all animals of the implant groups with implantation times of 5, 8, and 15 months, respectively (Figure 5). The newly formed tissue within the devices consisted of vascularised connective tissue containing fibromuscular cells surrounded by extracellular matrix. There was no cell metaplasia or necrosis, nor were there signs of calcifications locally related to the implants.
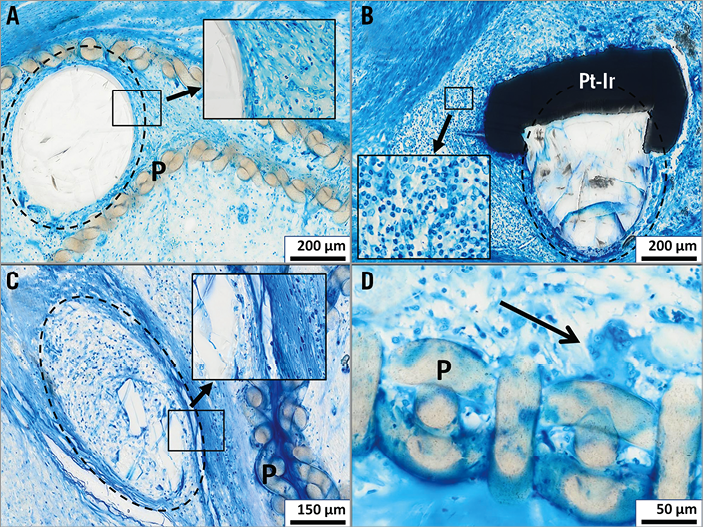
Figure 5. Bioresorption/inflammation. Representative histologic images demonstrating timely bioresorption of PLGA filaments (encircled by dotted line); no significant resorption of a PLGA filament next to the polyester cover (P) after an implantation time of 3 months (panel A, detail showing absence of inflammatory cells at the PLGA-tissue interface); start of resorption after an implantation time of 8 months with a lymphocytic infiltrate next to a Pt-Ir marker (Pt-Ir) (panel B, detail showing lymphocytes at higher magnification); almost completed resorption and substitution by fibrous tissue after an implantation time of 15 months (panel C, detail showing absence of inflammatory cells at the PLGA-tissue interface); foreign body giant cell (panel D, arrow) locally related to the polyester material (P) after an implantation time of 8 months; Richardson’s staining.
Degradation of the PLGA filaments was demonstrated to proceed with time (Figure 5), with substitution of the polymer material by fibromuscular cells and extracellular matrix in a similar pattern compared to neighbouring tissue parts.
IMPLANT-RELATED INFLAMMATORY REACTIONS
Results of the grading of cellular inflammatory reactions are summarised in Table 1. No signs of acute inflammation (i.e., granulocytes) were seen in any of the specimens. Chronic inflammatory reactions consisted of a few lymphocytic infiltrations loosely distributed in the newly formed tissue components (Figure 5B) and multinucleated foreign body giant cells neighbouring the polyester fibres of the foldable polymer covers (Figure 5D).
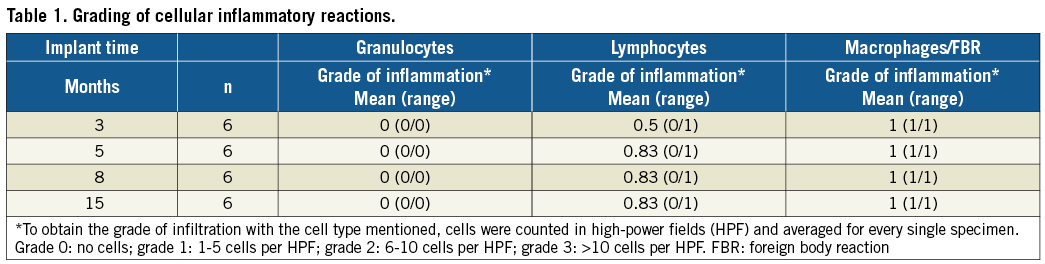
No inflammation was seen locally related to the PEEK filament holder or to the Phynox nuts. Accordingly, there was no cellular inflammatory reaction directed against the PLGA filaments independently of grade of biodegradation.
Discussion
In the literature, there is still much uncertainty concerning the long-term biocompatibility of permanent (non-resorbable) cardiovascular occlusion devices. Histologic in vivo data describe tissue reactions after an implantation time of four years at the most6,12. However, occlusion devices which are implanted in children will cause foreign body reactions for many decades. Fractures due to corrosion and bending forces, erosion or perforations of neighbouring structures, local thrombus formation, and calcifications have been described with uncertain and possibly negative implications for long-term biocompatibility6,7,13,14.
In contrast to their application in septal occlusion devices until now, bioresorbable materials have a long record in clinical use with proven good biocompatibility15,16.
Therefore, the CBSO was developed in order to reduce possible long-term complications by the use of bioresorbable materials, at least for the filament structure. Results of biocompatibility testing in an animal study are presented in this paper. In contrast, data on tissue reactions to metal mesh atrial septal defect (ASD) occlusion devices were never published prior to introduction into clinical use.
The first partially bioresorbable occluder implanted in humans was the BioSTAR (NMT Medical, Boston, MA, USA) with a metallic permanent framework (MP35N) and a bioresorbable membrane made from intestinal collagen17. Complications were reported with wire fractures and inflammatory reactions locally related to the biologic material of the membrane18. Meanwhile the BioSTAR occluder was withdrawn from the market. In contrast to the BioSTAR occluder, the CBSO has a framework with a bioresorbable filament structure. The results of the present study demonstrate a timely degradation process in vivo. Significant degradation with possible loss of filament stability was only reached in animals with an implantation time >8 months, when endothelialisation and tissue incorporation was shown to be completed.
Complete endothelialisation is critical for prevention of superficial thrombus formation in any kind of cardiovascular implant. In this study, we demonstrated fast and complete endothelialisation of the CBSO in an animal model. Furthermore, only a few inflammatory reactions were noted histologically without significant changes over time, which is considered beneficial for long-term biocompatibility1,11.
The use of completely bioresorbable septal defect occlusion devices in an experimental setting has been described previously. Wu et al and Duong-Hong et al reported on the follow-up four weeks after implantation of implants completely made of bioresorbable polymers in a swine model19,20. Complete endothelialisation and local unspecific inflammatory reactions were seen histologically. Zhu et al demonstrated partial degradation of polymer components of another biodegradable occluder in a canine animal model21. However, the observation period of that study was up to 24 weeks and therefore less than half the implantation time of the current study. The histological findings of all three studies correspond well with our findings in terms of endothelialisation, inflammatory reactions, and tissue proliferation.
In contrast to the previously mentioned devices, and after completion of the animal study, the CBSO has been introduced into clinical use in the meantime with promising short- and medium-term results with a complete ASD closure in 6/7 patients 12 to 24 months after CBSO implantation. For application in humans, one change in construction was made compared to the device as shown in Figure 1. The number of radiopaque markers was reduced from 32 to 16. The remaining markers are attached to the bioresorbable filaments and to the polyester covers and are located within these polyester covers in order to prevent embolisation of the Pt-Ir parts more securely during the degradation process.
For safety reasons and for promotion of endothelialisation, the CBSO has a polyester cover to prevent embolisation of possible framework fragments as stated above. This outer cover is permanent in contrast to the filament structure of the occluder. In view of the favourable biocompatibility demonstrated in this study, the question of whether the polymer cover could be substituted by a bioresorbable membrane might be discussed. This would result in a completely bioresorbable septal defect occlusion device with only radiopaque markers, if needed, being left behind.
Limitations
It has been demonstrated that biologic reactions to the septal defect occluder in an experimental setting and after human use show a high grade of correspondence6. Nevertheless, some uncertainty remains concerning the transferability of experimental results to the human organism. In addition, findings in animal studies are generally limited due to their relatively short observation periods compared to the expected implantation time in humans.
Conclusions
In summary, this study demonstrated the good biocompatibility of the Carag Bioresorbable Septal Occluder. Nevertheless, it is still the final aim to create a device with no long-term reactions by avoiding the use of permanent materials altogether. The development of the CBSO is an important step towards the aim of a completely bioresorbable occlusion device.
| Impact on daily practice This is the first report on histopathology of a septal defect occluder with a bioresorbable filament structure in vivo which is already in clinical use. Tissue reactions such as endothelialisation, inflammatory reactions, tissue proliferation, and the process of degradation of the bioresorbable components are described in detail and with high-quality figures. |
Acknowledgements
The authors thank Maren Hasper, Andrea Poppe, and Karin Bär for technical assistance.
Funding
The GLP study was funded by CARAG AG. No other funding sources were used.
Conflict of interest statement
M. Sigler performed the histopathologic evaluation of results as a contract investigation for CARAG AG. Interpretation of the results was not influenced by employees of the company. M. Sigler has no further financial relationship with CARAG AG or any other conflict of interest. B. Söderberg and B. Schmitt are research consultants to CARAG AG. They have no further financial relationship with the company or any other conflict of interest. A. Mellmann and J. Bernhard are employees of CARAG AG. They have no other conflicts of interest.

