Abstract
Background: An atrial septal occluder (ASO) represents a major obstacle to the widespread adoption of atrial fibrillation (AF) catheter ablation in patients with prior atrial septal defect (ASD) closure.
Aims: The aim of this study was to describe the “sequential technique” of transseptal puncture (TSP) in AF patients with ASO.
Methods: Sixty-four drug-refractory AF patients with ASO who underwent catheter ablation in our centre from September 2007 to March 2020 were enrolled.
Results: Puncture through the native septum was achieved in 29 patients (Group A) and through the device in 35 patients (Group B). The mean diameter of the occluder was significantly larger in Group B than in Group A (31.6±4.6 mm vs 22.8±3.5 mm, p<0.001). The mean time of TSP (24.9±8.8 vs 5.8±2.1 min, p<0.001), total fluoroscopy time (23.7±10.9 vs 7.5±4.4 min, p<0.001), and total procedure time (172.7±58.3 vs 123.4±43.8 min, p=0.001) of Group B were significantly longer than those of Group A. In Group B, the external sheath crossed the device by reshaping the needle and adjusting the puncture angle and position in 23 patients (Group B1), while the external sheath crossed the device with the assistance of balloon dilation in 12 patients (Group B2). No patient had thrombus, periprocedural interatrial shunt or procedural complications.
Conclusions: TSP and AF ablation in patients with ASO are feasible and safe. The “sequential technique” could be safely used in patients with ASO.
Introduction
Patients with atrial septal defects (ASD) are at high risk of developing atrial fibrillation (AF)12345. There is an increasing number of patients undergoing transcatheter closure of ASD who subsequently develop AF345. Catheter ablation has developed as an efficient therapeutic approach for AF over the past decade67. Transseptal access to the left atrium (LA) is a prerequisite for the ablation, and transseptal puncture (TSP) in patients with atrial septal occluder (ASO) devices is likely to be required with increasing frequency. However, the ASO makes the TSP more difficult and risky, which represents a major obstacle to the widespread adoption of catheter ablation of AF in patients with prior ASD closure8. It has been reported that transoesophageal echocardiography (TOE) or intracardiac echocardiography (ICE) could be used for TSP in patients with ASO89. We have previously described the balloon dilation approach facilitating TSP through occluders10. Nonetheless, their routine clinical use might be precluded by extra costs and manpower. In this study, we summarise our improved experience and describe the “sequential technique” of TSP in patients with ASO for AF catheter ablation.
Methods
Study population
From September 2007 to March 2020, sixty-four consecutive drug-refractory AF patients with ASO undergoing catheter ablation were included. Exclusion criteria included: (1) percutaneous ASD closure performed within 6 months; (2) patent foramen ovale; (3) LA anterior-posterior diameter >55 mm; (4) left ventricular ejection fraction <35% or New York Heart Association cardiac function III or IV; and (5) left atrial thrombus. All antiarrhythmic drugs except for amiodarone were discontinued at least five half-lives before the procedure. TOE was performed before the procedure in all patients to exclude left atrial thrombus. Long-term therapeutic administration of warfarin or new oral anticoagulants (NOACs) was not discontinued during the perioperative period. The study protocol was approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University. Written informed consent was obtained from all patients.
Transseptal puncture
Evaluation of anatomic structures
A planning preprocedural cardiac computed tomography (CT) scan or an intraprocedural ICE was performed to identify the native atrial septum and to study the anatomic relationships among the septum, right atrium (RA), LA, aortic root and the device. The decision as to whether to undertake TSP on the native septum or the device was made based on the evaluation of the anatomic structures. If there was a portion of native septum connected between the LA and RA suitable for the TSP, the puncture was achieved via the native septum beside the ASO. Direct puncture through the ASO was performed when the closure device occupied the whole septum or after a failed attempt on the native septum (Figure 1).
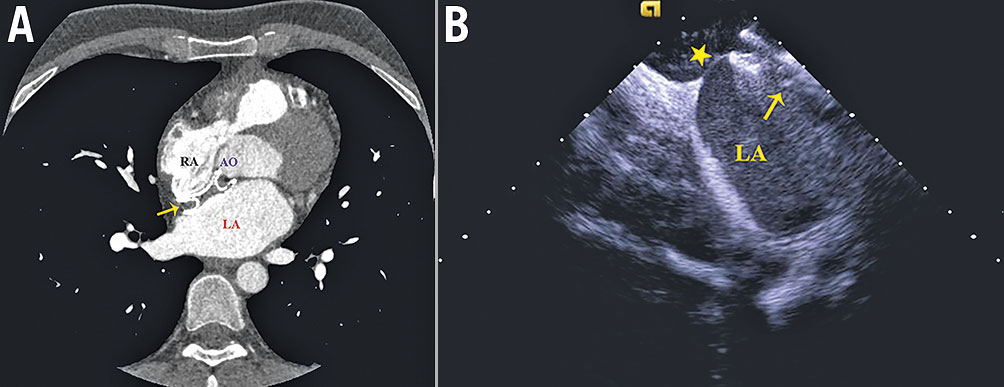
Figure 1. Preprocedural cardiac computed tomography (CT) scan and intraprocedural ICE to identify the anatomic relationships among the septum, RA, LA, AO and the device. A) Preprocedural CT image showed no suitable native septum for the TSP. B) ICE during the procedure showed the native septum (asterisk) posteroinferior to the device suitable for the TSP after careful examination. The arrow indicates the atrial septal occluder. AO: aortic root; ICE: intracardiac echocardiography; LA: left atrium; RA: right atrium
Transseptal puncture through the native septum
A steerable decapolar was positioned within the coronary sinus through the left femoral vein. Left atrial access was obtained with a single TSP guided by fluoroscopy. An 8.5 Fr transseptal sheath and a dilator (SL1; St. Jude Medical) were advanced over a 0.032-inch guidewire to the superior vena cava (SVC). Then the guidewire was removed, and a Brockenbrough transseptal needle (St. Jude Medical) was introduced into the dilator and advanced to just proximal to the sheath tip. On the fluoroscopic anteroposterior view, an entire apparatus comprised of the sheath, dilator and transseptal needle was oriented towards the interatrial septum (4 to 5 o’clock position) and withdrawn down the SVC and interatrial septum smoothly until the dilator tip “jump” was observed, which indicated the site of the device or the fossa ovalis (FO) located around the ASO. On the right anterior oblique 45° (RAO 45°) view, the transseptal assembly was slightly rotated clockwise to the posterior of the device or counterclockwise to the anterior. Then the needle was advanced beside the device across the native septum. After a small amount of contrast material had been injected to confirm that the needle was in the proper location, the dilator was advanced over the needle into the LA. The ablation catheter (NAVISTAR THERMOCOOL; Biosense Webster) was then advanced to the LA after the external sheath had entered into the LA.
Transseptal puncture through the device
Owing to the presence of the ASO, the engagement of the FO could barely be assessed by the fluoroscopic visualisation of a “fall” to the septum. On the anteroposterior view, the transseptal assembly was gently withdrawn from the SCV to the centre of the device. On the RAO 45°, the needle was advanced into the LA through the occluder. Then the dilator was advanced to cover the tip of the needle. The external sheath was advanced into the LA over the dilator and the needle.
Once the external sheath met difficulties in crossing the device, the transseptal needle was withdrawn and reshaped into a larger curve in vitro. Then the process was performed again, and the needle was adjusted to a proper position to make sure that the needle tip was almost perpendicular to the plane of the occluder (Figure 2). In the vertical position to the plane of the occluder, the needle might traverse the device. After confirming the LA access by contrast injection, the inner dilator was pushed to cover the tip of the needle. Then the external sheath was pushed across the ASO device into the LA over the dilator.
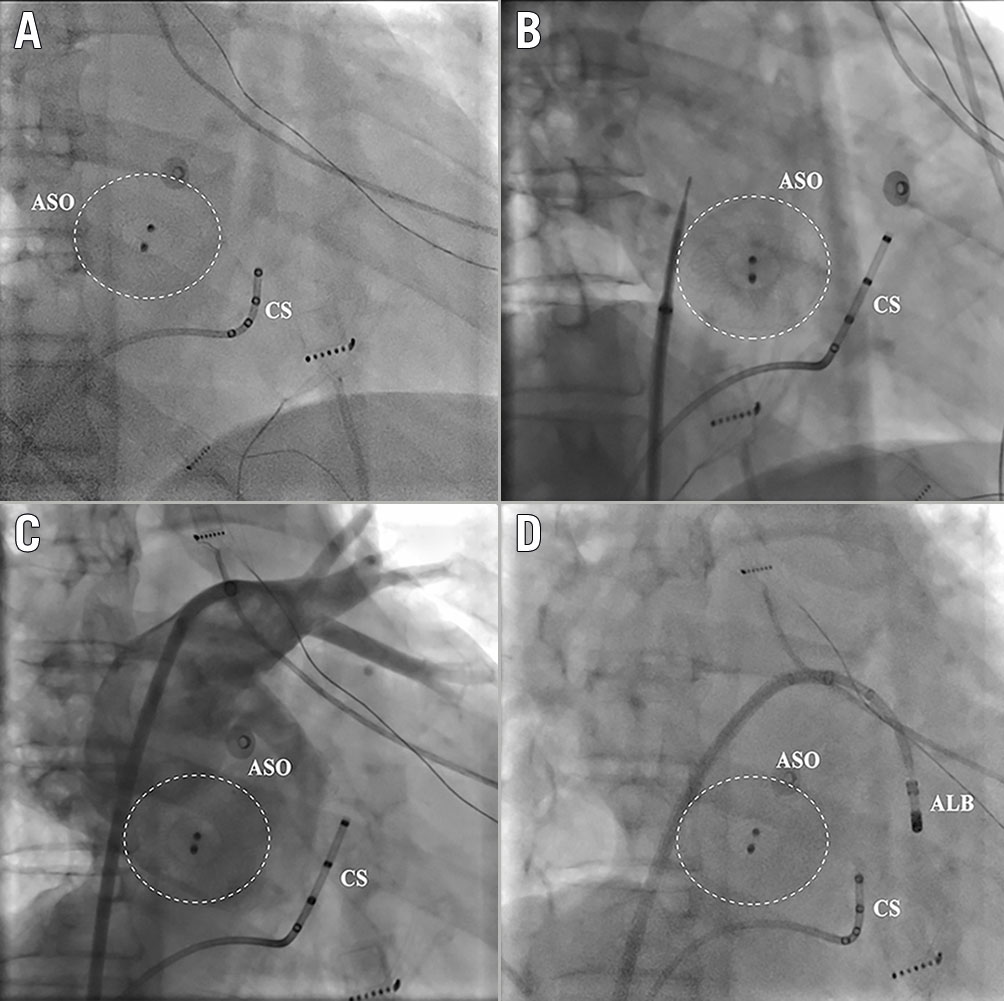
Figure 2. Puncture through the native septum. Puncture through the native septum posteroinferior to the implanted ASO. A) The position of the ASO in the right anterior oblique 30° view. B) The puncture position of the needle was on the native septum posteroinferior to the ASO. C) The angiograpy of the left atrium showed the relationship of the puncture position with the ASO. D) The ALB advanced through the external sheath into the left atrium. ALB: ablation catheter; ASO: atrial septal occluder; CS: coronary sinus
If the external sheath still failed to cross the device even after several attempts, the balloon dilation approach was used to assist the passage of the external sheath, as we described previously10. The transseptal needle was then removed and a 0.014-inch×190 cm BMW guidewire (Abbott) was advanced through the dilator into the LA and advanced into the left superior pulmonary vein. Then the dilator was withdrawn and the 3.0-5.0 mm non-compliant rapid-exchange angioplasty balloon (VOYAGER NC; Abbott) was advanced across the device to enlarge the access site further with an inflation pressure of 14-18 atm. After this, the external sheath could be advanced easily into the LA.
Mapping and ablation
The electrophysiological study and ablation procedure were performed under conscious sedation with fentanyl and midazolam, as previously described1112. A bolus of unfractionated heparin (7,000 U) was administered after the transseptal access, and intermittent infusion was adopted to maintain an activated clotting time (ACT) >300 s. The ablation strategy for patients with paroxysmal AF was circumferential pulmonary vein isolation (CPVI), whereas, for persistent AF, linear ablations at the LA roof, mitral isthmus (intra-CS when necessary), and cavotricuspid isthmus were added12. If the patient was not in sinus rhythm after initial ablation, cardioversion was performed. The endpoint was CPVI and bidirectional block when linear ablation was performed for paroxysmal and persistent AF, respectively. Radiofrequency ablation was carried out in a power-controlled mode with 25 to 45 W and with a saline irrigation speed of 17 mL/min (30 mL/min when ablating in the coronary sinus).
Follow-up
After ablation, anticoagulation and antiarrhythmic drug therapy (ADT) were continued for three months. At three months, anticoagulation was continued according to the stroke risk, whereas ADT was continued on the decision of the treating physician. The first three months were set as the blanking period. Transthoracic echocardiography (TTE) was performed to detect thrombus, residual interatrial shunt or device deformation both on the day after the ablation procedure and three months after the procedure. Office or transtelephonic visits were scheduled at 3, 6, and 12 months following the procedure and every 6 months thereafter. A 12-lead Holter was performed at each office visit. Recurrence was defined as any episode of AF or atrial tachycardia lasting for at least 30 seconds after a blanking period.
Statistical analysis
Continuous variables were expressed as mean±SD and compared by the Student’s test. Categorical variables were expressed as percentages (%) and compared by chi-square analysis or Fisher’s exact test, as appropriate. A p-value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS, Version 26.0 (IBM Corp.).
Results
Clinical characteristics
Sixty-four patients (age 50.8±10.3 years, 28 male) with drug-refractory symptomatic AF and percutaneous ASD closure devices (Amplatzer Septal Occluder [Abbott]; Lifetech ASD Occluder [Lifetech Scientific]) were included in the study. The device had been implanted for an average of 136 months (range 9-468 months). Seven patients had a history of AF before the percutaneous device closure (range 0.2-466 months, mean time before the closure procedure of 119.8±114.2 months), in three patients AF had occurred during the percutaneous device closure procedure, and the others developed AF after the closure procedure (range 3-84 months, mean time after the closure procedure of 24.6±28.1 months). Catheter ablation was performed in 43 patients with paroxysmal AF and 21 patients with persistent AF. The baseline clinical characteristics of the participants are presented in Table 1.
Transseptal access and procedural data
Transseptal access and procedural data are shown in Table 2. All the patients underwent CT before the procedure; in two of them ICE was used during the procedure. Single transseptal access was successfully obtained in all 64 patients. Puncture was achieved through the native septum in 29 patients (Group A, paroxysmal AF 20 and persistent AF 9) and through the device in 35 patients (Group B, paroxysmal AF 23 and persistent AF 12). One patient had failed TSP because the needle entered the aorta, precluding further ablation, and that patient was excluded from the present analysis.
In Group A, an interatrial site posteroinferior to the implanted ASO was targeted in 26 patients (Figure 2), and in three patients an interatrial site anteroinferior to the implanted ASO was targeted.
In Group B, the external sheath crossed the device by reshaping the needle and adjusting the puncture angle and position to make the needle nearly vertical to the device plane in 23 patients (Group B1) (Figure 3). The external sheath crossed the device with the assistance of balloon dilation in 12 patients (Group B2) (Figure 4).
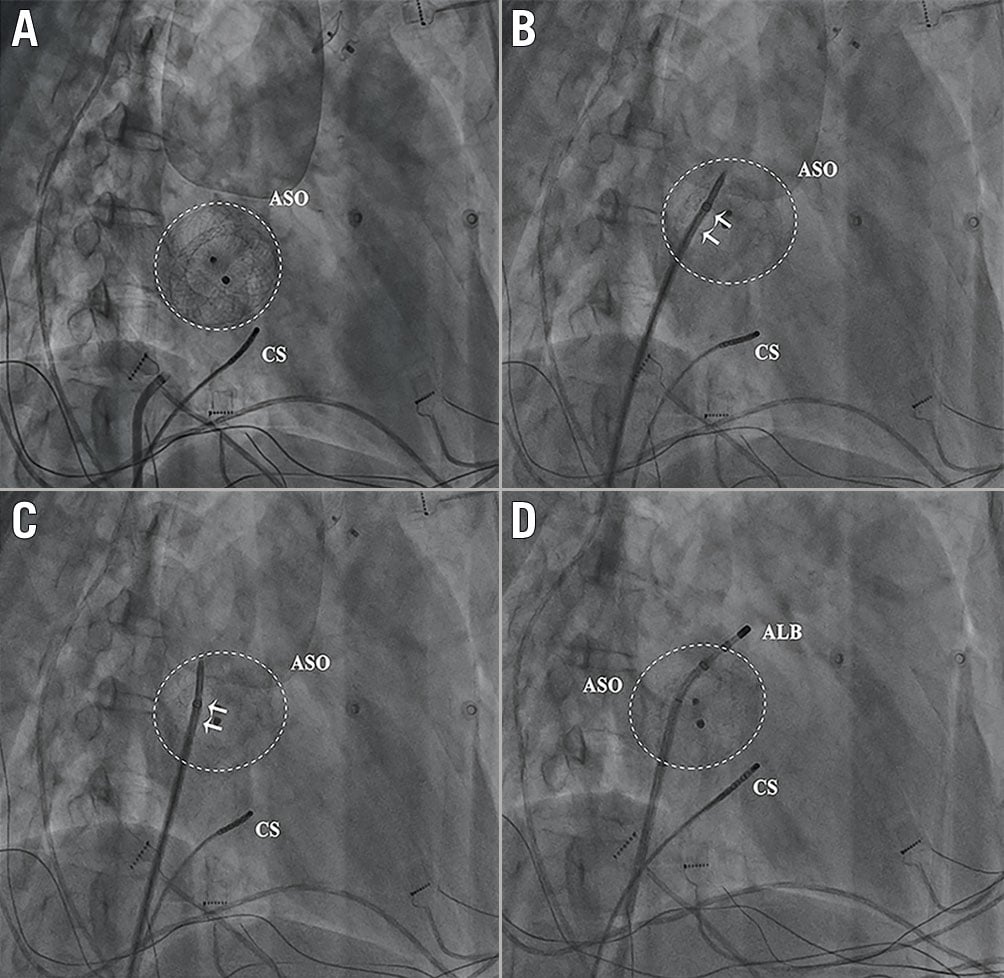
Figure 3. Puncture through the ASO and the external sheath crossing the device only by reshaping the needle and adjusting the puncture angle and position to make the needle nearly vertical to the device plane. A) The position of ASO in the right anterior oblique 30° view. B) The external sheath could not cross the device even with a large pushing force (arrow). C) & D) After reshaping the needle and adjusting the puncture angle and position, the external sheath crossed the device easily. ALB: ablation catheter; ASO: atrial septal occluder; CS: coronary sinus
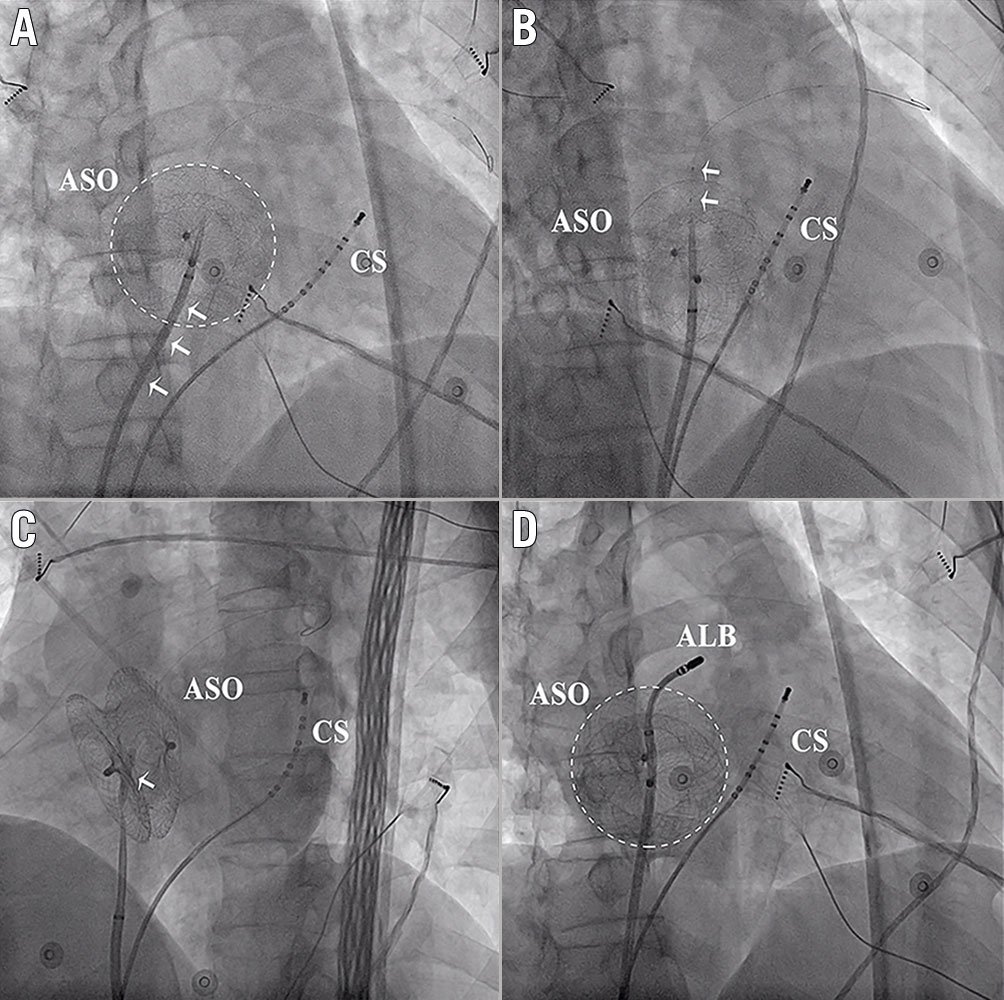
Figure 4. Puncture through the ASO and the external sheath crossing the device with the assistance of balloon dilation. A) The external sheath could not cross the device even with a large pushing force. B) & C) Balloon dilation (arrows indicate the balloon). D) After the balloon dilation, the external sheath crossed the device easily. ALB: ablation catheter; ASO: atrial septal occluder; CS: coronary sinus
The mean diameter of the occluder was significantly larger in Group B than in Group A (31.6±4.6 mm vs 22.8±3.5 mm, p<0.001). The mean time of TSP (24.9±8.8 vs 5.8±2.1 min, p<0.001), total fluoroscopy time (23.7±10.9 vs 7.5±4.4 min, p<0.001), and total procedure time (172.7±58.3 vs 123.4±43.8 min, p=0.001) of Group B were significantly longer than those of Group A. Additionally, the procedure time after removal of the TSP duration of Group B was also significantly longer than that of Group A (147.8±54.0 vs 117.6±43.7 min, p=0.018). This might have been due to the progressively tougher manipulation of the catheter resulting from the elastic retraction of the occluder in Group B. The mean time of TSP (30.5±9.9 vs 22.0±6.7 min, p=0.005), total fluoroscopy time (30.6±13.8 vs 20.1±7.0 min, p=0.027), and total procedure time (202.3±63.8 vs 157.3±49.9 min, p=0.028) of Group B2 were significantly longer than those of Group B1.
The procedure endpoint was achieved in all 64 patients (Table 2). No complications occurred during the ablation procedure, including haemopericardium, thromboembolic events, groin haematoma, vascular complication and bleedings. No patient had evidence of periprocedural interatrial shunt.
Follow-up
At the follow-up three months after the ablation, the echocardiography showed no thrombus or interatrial shunt in any of the patients. After a follow-up of 61.5±42.4 months, sinus rhythm was maintained in 23 patients (79.3%) in Group A and 25 patients (71.4%) in Group B (p=0.47). Sixteen patients had a recurrence of AF. No procedure-related complications, including haemopericardium, thromboembolic events, groin haematoma, vascular complication, bleedings, atrial-oesophageal fistula and pulmonary vein stenosis, were observed.
Discussion
Main findings
To our knowledge, the present study reports the largest series of patients undergoing AF catheter ablation with ASO. The main findings are the following. (1) For AF patients with ASO, TSP could be achieved through either the occluder device or the native septum. Evaluation of the CT image before the procedure is of great importance. (2) Needle reshaping and adjustment of the puncture position and angle between the needle and device plane could facilitate the passage of the external sheath through the ASO in the majority of patients. (3) Although the manipulation of sheath and catheter is challenging, TSP and ablation are safe and effective for patients with ASO when undertaken by experienced operators.
Feasibility and safety of AF ablation for patients with ASO
Patients with ASD experienced an increased incidence of AF because of the prolonged volume overload with atrial stretch. Catheter ablation has become an effective treatment approach for AF. However, data on the feasibility and safety of AF ablation after percutaneous closure of ASD are rare. Some experienced centres have reported the safety and feasibility of AF catheter ablation in these patients81013. Li et al performed AF ablation in nine patients with a percutaneous ASD closure device; successful TSP and ablation were achieved in all of the patients13. Santangeli et al included 34 AF patients with a percutaneous ASD closure device and found that ablation was feasible, safe, and effective in them8. No patient in these studies showed any major procedural complications, including device dislodgement, which is consistent with our experience1014. Thus, it is reasonable and safe to perform AF ablation in patients with an ASO.
Our experience suggests that TSP through the native septum next to the ASO could be considered if the device diameter is ≤28 mm. Direct puncture through the device may be necessary when the device is oversized in relation to the interatrial septum, especially when the device diameter is >28 mm. In one patient in our study, TSP was performed through the native septum on the RA side and across the ASO on the LA side with the assistance of balloon dilation (Figure 5). Even with the puncture only through the left disc, the external sheath still hardly crosses the disc, which might result from the angle of the puncture. Hence, we do not recommend performing the puncture through the single disc because it might cause difficulties for the subsequent manipulation. It is important to perform a CT image to evaluate the anatomic relationship before the ablation procedure. The evaluation of the anatomic relationship with ICE during the procedure is also of great importance, especially for patients with occluders of critical diameter, often with a diameter of 26 to 28 mm. In these patients, the CT image frequently shows no native septum suitable for the puncture; however, the ICE might reveal a suitable native septum for the puncture after careful examination during the procedure (Figure 1).
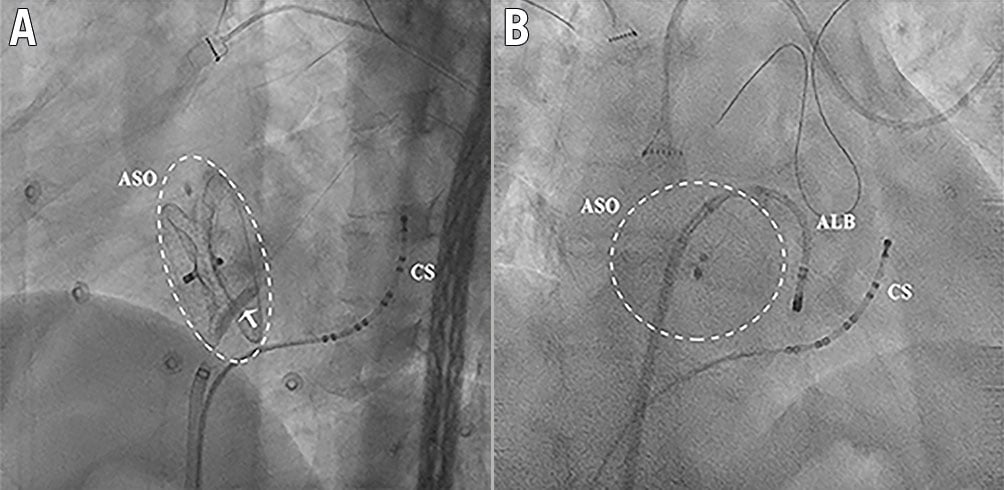
Figure 5. Puncture through the ASO only on the left side of the device and the external sheath crossing the device with the assistance of balloon dilation. A) Balloon dilation only on the left side of the ASO (arrow indicates the balloon). B) After the balloon dilation, the ALB could be manipulated easily. ALB: ablation catheter; ASO: atrial septal occluder; CS: coronary sinus
It has been demonstrated that the ASO was completely covered with protein and cellular layers within approximately three months after percutaneous closure of ASD15. Thus, transseptal access through the device is unlikely to damage or deform the highly resilient and elastic polymer mesh which constitutes the closure device. This may also explain the absence of detectable shunt post-procedure8. At the same time, the highly resilient and elastic polymer mesh might make catheter manipulation progressively tougher during the procedure. Furthermore, it is important to emphasise that six months should be allowed after device closure before attempting AF ablation16.
“Sequential technique” of TSP in patients with ASO
The major obstacle to the widespread adoption of AF catheter ablation in patients with ASO is the perceived higher risk and technical challenge of transseptal access in the presence of an occluder device. Different transseptal accesses used in these patients have been reported8101314. In the majority of patients transseptal access in the native septum with small size occluder devices could be obtained. In a minority of patients with large size devices, TSP could be safely performed directly via the ASO because of the insufficient rims of the native septum for safe transseptal access1013. In the present study, TSP was usually performed directly through the device if the device diameter was >28 mm. Because catheter manipulation might become progressively tougher with elastic retraction of the occluder, the native septum is preferred for the TSP. If the attempted TSP through the native septum fails, TSP through the ASO could also be considered. Before an attempted TSP, it is of great importance to identify the relationship between the native interatrial septum and the neighbouring structures by CT imaging. For patients with ASO of critical diameters (often 26-28 mm), ICE during the procedure is also helpful to evaluate the structures and to find a suitable native septum for TSP.
The high level of resistance of the occluder usually prevents the external sheath from crossing the device. Santangeli et al reported on the technical feasibility of direct access through the device using an upsized dilator for AF ablation under ICE guidance8. Additionally, balloon dilation was often used to facilitate TSP through a fibrotic interatrial septum101314. Our initial experience also suggested that balloon dilatation could facilitate the transseptal passage. However, the process and manipulation were still cumbersome. In this study, we improved our initial experience of TSP in patients with ASO. According to the experience of our centre, in the majority of cases, the external sheath could directly cross the device without additional dilation of the puncture site. Needle reshaping and adjustment of the puncture angle and position are of great importance. To facilitate the passage of the external sheath, it is necessary to make the puncture needle nearly vertical to the plane of the device by reshaping the needle and adjusting the angle and position of the puncture (Figure 6). In this way, the external sheath could cross the ASO directly without extra dilation in most cases. If the external sheath still cannot pass the ASO after several attempts, balloon dilation should be considered. The Central illustration summarises the “sequential technique” of TSP in patients with ASO during catheter ablation of AF.
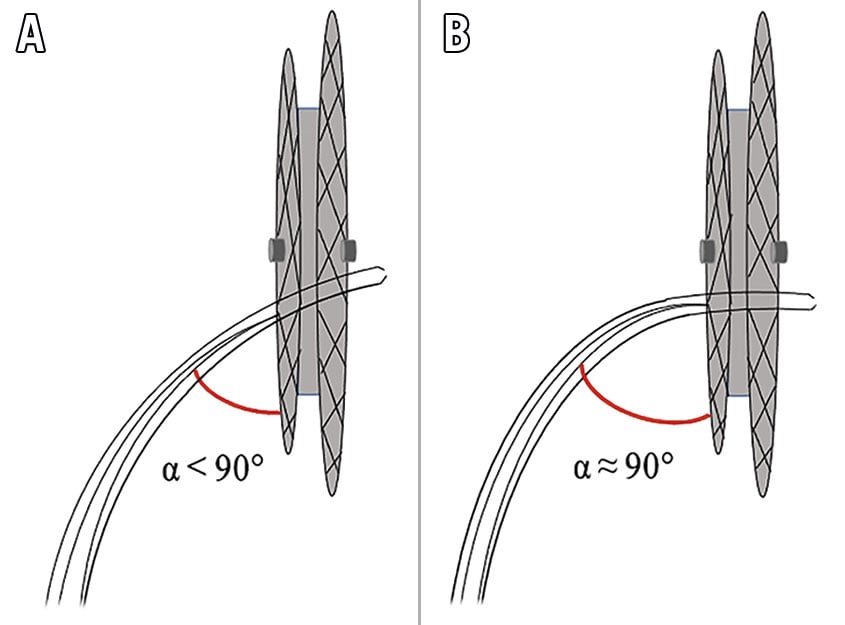
Figure 6. The appropriate angle for the external sheath to pass the occluder. If it is difficult to make the external sheath pass the occluder with an angle smaller than 90° (A), reshaping the needle and adjusting the puncture angle to make the needle nearly vertical to the device plane might facilitate the passage of the external sheath (B).
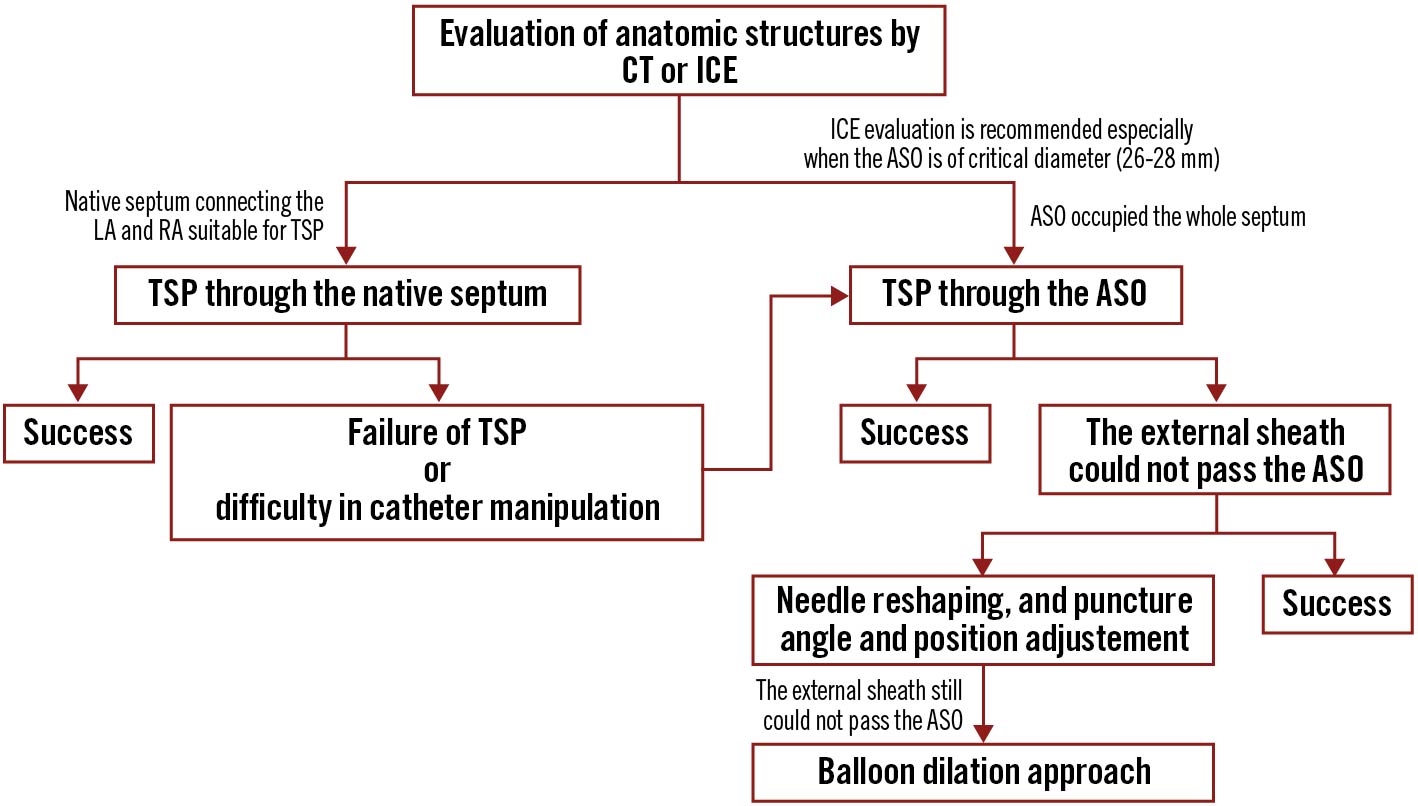
Central illustration. The “sequential technique” of transseptal puncture. The diagram shows the “sequential technique” of transseptal puncture in patients with atrial septal occluders. ASO: atrial septal occluder; LA: left atrium; RA: right atrium; TSP: transseptal puncture
Study limitations
The study design was retrospective; ICE was not used to guide the TSP in every patient. Thus, in some patients in the study with relatively large occluders, TSP might also have been achieved through the native septum instead of the ASO, especially for those with an ASO of diameter between 26 and 28 mm. Additionally, the proportion of TSP through the occluder was higher in our study than in other studies. This might have been because the majority of patients in our study were referred by other centres due to the challenge of TSP and ablation in these patients.
Conclusions
TSP and AF ablation in patients with ASD closure devices are feasible and safe. TSP was achieved both in the native septum and directly through the device. The “sequential technique” can facilitate the TSP and be safely and systematically used in AF patients with ASD closure devices.
Impact on daily practice
Peri-device transseptal puncture (TSP) can be considered when the diameter of the atrial septal occluder (ASO) is ≤28 mm. Needle reshaping and the adjustment of the puncture position and angle to make the needle nearly vertical to the device plane could facilitate the passage of the external sheath through the ASO in the majority of patients. The “sequential technique” which is summarised from our improved experience could facilitate the TSP and be safely and systematically used in atrial fibrillation patients with ASO.
Funding
This work was supported by the National Key Research and Development Program of China (2016YFC0900901 to Dr Ma, 2018YFC1312501 to Dr Bai, and 2017YFC0908803 to Dr Tang).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

