Abstract
A number of interventional procedures based on the transseptal puncture (TSP) have been developed in recent years. The increasing number of interventional procedures, as well as the use of large-bore sheaths and complex devices, has led to improvements in technique and equipment. The combined use of fluoro-scopy and of transoesophageal echocardiography (TEE) has increased safety and precision. However, TSP still represents a tricky procedure, which may become even more difficult in cases of challenging interatrial septa, and life-threatening complications may occur. Consequently, an in-depth knowledge of procedural steps, equipment, echocardiographic views, fossa ovalis anatomy and how to manage the most frequent complications is critical to performing a successful TSP.
Introduction
The transseptal puncture (TSP) technique was introduced into clinical practice during the late 1950s by Ross et al as a diagnostic tool for proper selection of patients for cardiac valve surgery1,2. Over the years, its use for diagnostic purposes has declined. However, a number of new interventional procedures utilising TSP have been developed, such as electrophysiology ablations, percutaneous mitral valve repair/implantation, patent foramen ovale closure, left atrial appendage occlusion, paraprosthetic valve leak repair and left ventricular assist device positioning.
As anatomic landmarks are not visible under fluoroscopy, the use of echocardiography has become essential to increase safety and allow a more precise detection of the crossing-point location within the atrial septum.
To achieve a successful procedure and to avoid complications, an in-depth knowledge of interatrial septum (IS) anatomy, available equipment, correct imaging guidance, and procedural steps is needed. This review will describe fluoro-echo-guided TSP steps and equipment, providing useful tips and tricks and a guide to complications in order to manage the most challenging anatomies and complications.
EQUIPMENT
The initial needle used by Ross had a curved distal end to allow controlled movements of the tip and an arrow-shaped proximal handle to define needle orientation. Although design features have remained the same over the years, Brockenbrough modified the Ross needle by reducing the calibre of the distal 1.5 cm of the needle from 18 to 21 gauge3,4.
The Brockenbrough (BRK) needle is made of stainless steel and has a stylet inside the lumen to prevent friction while advancing it into the sheath. The standard BRK needle (St. Jude Medical, St. Paul, MN, USA) has a 19° angle between the distal curved part and the needle shaft, whereas the BRK 1 (St. Jude Medical) is characterised by a 53° angle curvature. However, the needle curve can be hand shaped. Paediatric needles are also available with different lengths and curves.
Several sheaths with a dilator protruding beyond the sheath tip can be used with BRK needles. Nowadays, several manufacturers produce transseptal kits, although they have only slight vari-ations in design (Figure 1). The most common sheaths are the Mullins sheath and the Swartz™ Braided Superior Left (SL) (St. Jude Medical), both of which are available with different bores (8-12 Fr) and different curvature versions according to atrial anatomic features. In general, the Braided SL has more pushability and torquability compared to the Mullins sheath and its curvature is suitable for most procedures as it points at the superior left pulmonary vein, whereas the Mullins sheath has an ideal curve to reach the mitral valve.
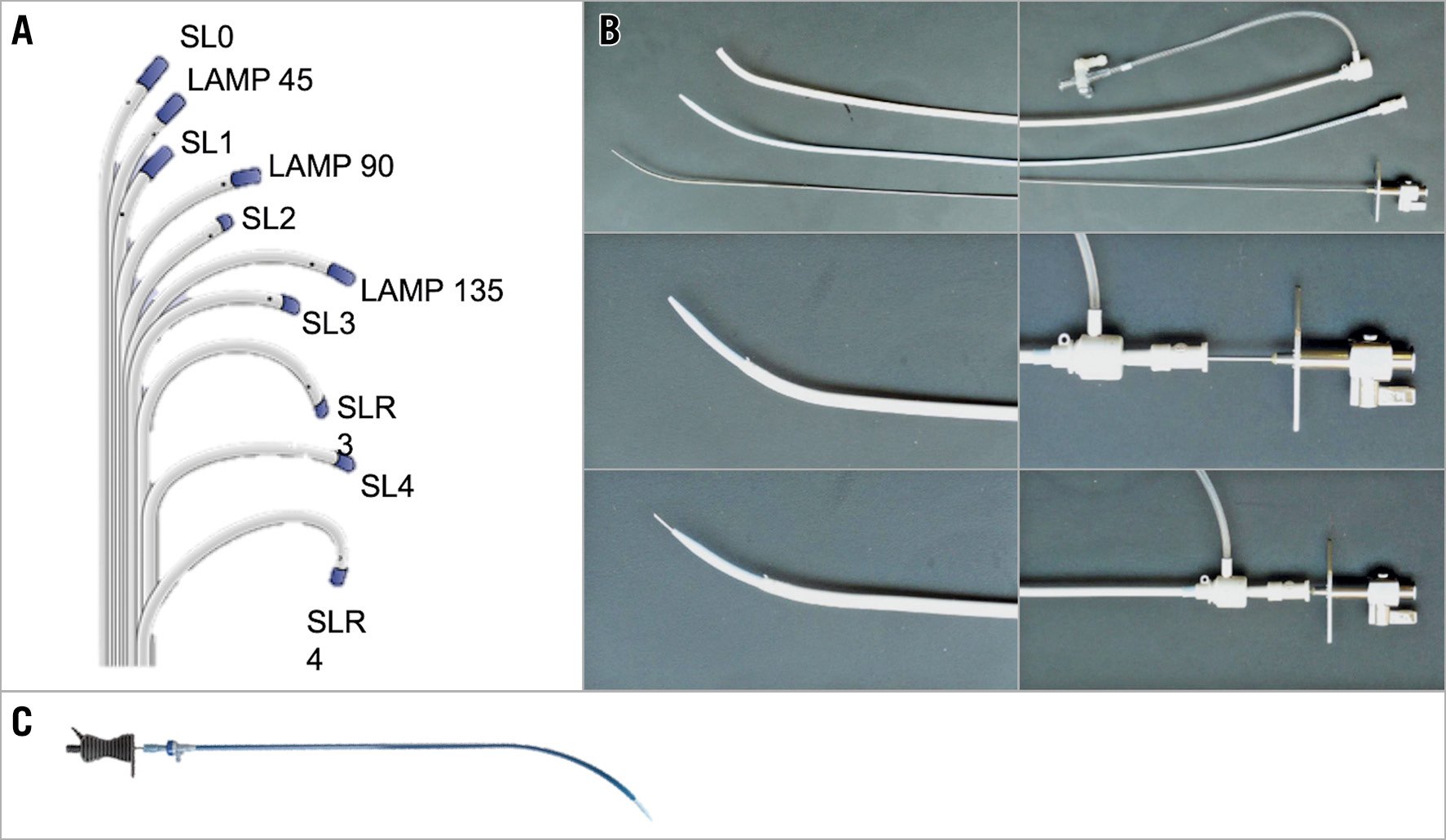
Figure 1. Transseptal kits. A) Swartz SL transseptal sheaths. B) St. Jude transseptal kit. C) NRG radiofrequency transseptal needle (Baylis Medical).
FOSSA OVALIS AND ATRIAL SEPTUM ANATOMY
The anatomic interatrial septum (IS) is the entire area interposed between the two atria and should be distinguished from the “true” IS, which is only the area that can be crossed without going into the extracardiac space and accounts for only approximately 20% of the total septal area5.
The fossa ovalis is part of the true IS and is located in the lower posterior part of the IS. It is either an oval or, less frequently, a round depression, composed primarily of thin fibrous tissue. The fossa ovalis can be schematically divided into four parts (Central illustration, A) – (1) superior-anterior, (2) inferior-anterior, (3) superior-posterior, and (4) inferior-posterior.
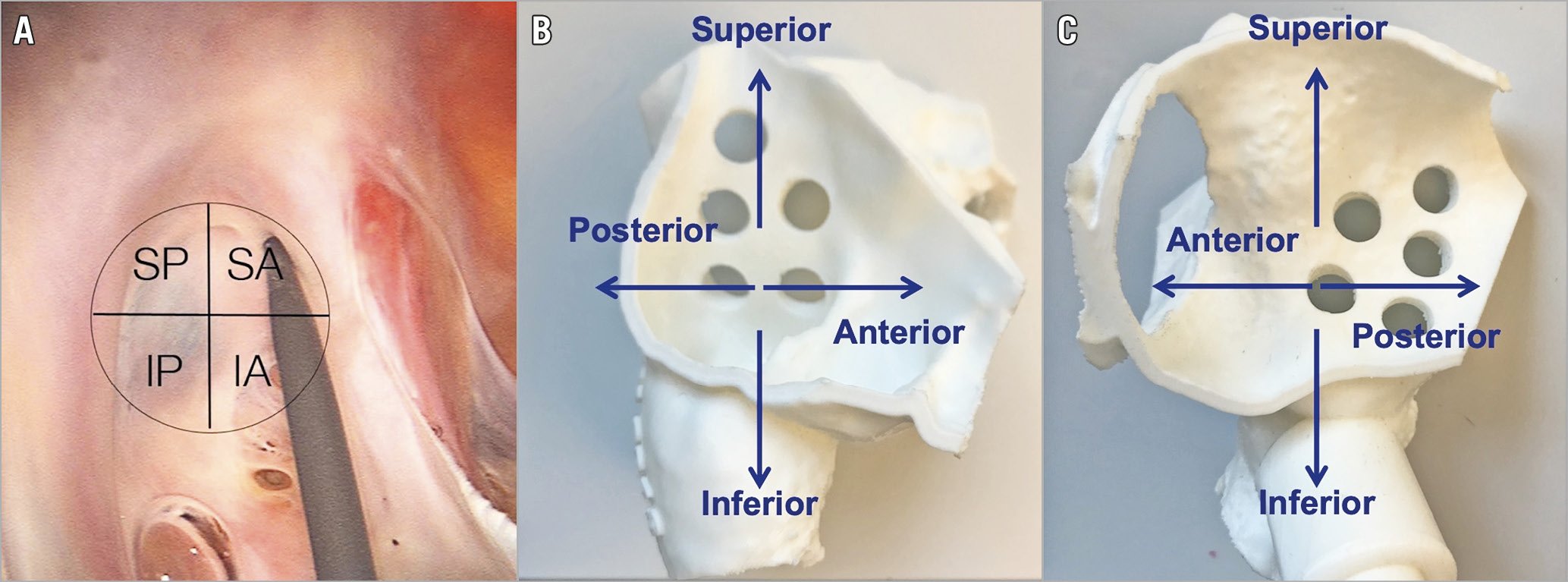
Central illustration. Fossa ovalis anatomy from the RA view and its schematic division into four parts (A). Three-dimensional models showing the IS from the right side (B) and the left side (C) and its relationship with the mitral valve plane.
Its shape may vary significantly according to atrial size, pressure, and tissue redundancy. The three-dimensional reconstruction in the Central illustration, B & C shows different puncture sites within the right and left sides of the fossa ovalis and their relationship with the mitral valve.
PREPROCEDURAL IMAGING ASSESSMENT
Preprocedural transoesophageal echocardiography (TEE) is recommended in all patients undergoing TSP because it provides useful insights into the IS anatomy (e.g., patent foramen ovale, atrial septal defects [ASDs] or challenging septa) and allows detection of left atrial thrombus or atrial tumours, which represent contraindications to TSP. Preprocedural computed tomography (CT) is usually indicated for transcatheter mitral valve implantation (TMVI) or for some mitral repair procedures (e.g., transcatheter direct annuloplasty), providing fundamental information such as aortic-mitral valve angle, neo-left ventricle outflow tract and device placement simulation for TMVI, or distance between the annulus and coronary vessels for direct annuloplasty. Some software also allows the assessment of the fossa ovalis and the anatomical relations between IS, left atrial appendage ostium, mitral annulus and vena cava. In this way, catheter orientation, height from mitral annular plane and C-arm orientation for TSP can also be simulated and planned before the procedure6.
VASCULAR ACCESS-SITE MANAGEMENT
Venous femoral puncture represents the first step of the TSP procedure. Usually, a right-sided venous femoral puncture is preferred due to the linear trajectory to the right atrium (RA), as compared to the left route. However, both groins should be prepared, as the left side might be used for:
– invasive arterial monitoring
– venous/arterial access site in case of any complication
– TSP access site in case of unfavourable right venous axis ana-tomy. In such cases, the left side is to be considered although catheter trackability may be reduced in which case a more curved needle to reach the fossa will be required.
In order to achieve vascular haemostasis, a figure-of-eight stitch or, alternatively, preclosure with a closure device (Perclose ProGlide®; Abbott Vascular, Santa Clara, CA, USA) is used.
Tips and tricks
To achieve a safe femoral puncture, fluoroscopic and/or ultrasound guidance is strongly suggested. A low puncture is advisable because the femoral vein does not bifurcate, and it is a reasonable size until a few centimetres from the inguinal ligament. In this way, the risk of retroperitoneal bleeding is reduced. Echo guidance with a linear probe allows the operator to assess vein anatomy and its relationship with the femoral artery and to follow the needle direction while reaching the vein to avoid femoral artery puncture (Figure 2). In case of the unavailability of echo guidance, the femoral head represents the fluoroscopic landmark (anteroposterior [AP] projection): the puncture should be medial to it and below its equator. An alternative option is to palpate the femoral pulse and puncture 4 cm below the inguinal ligament, medial to the femoral pulse and with a 45° inclination starting medial and shifting lateral.
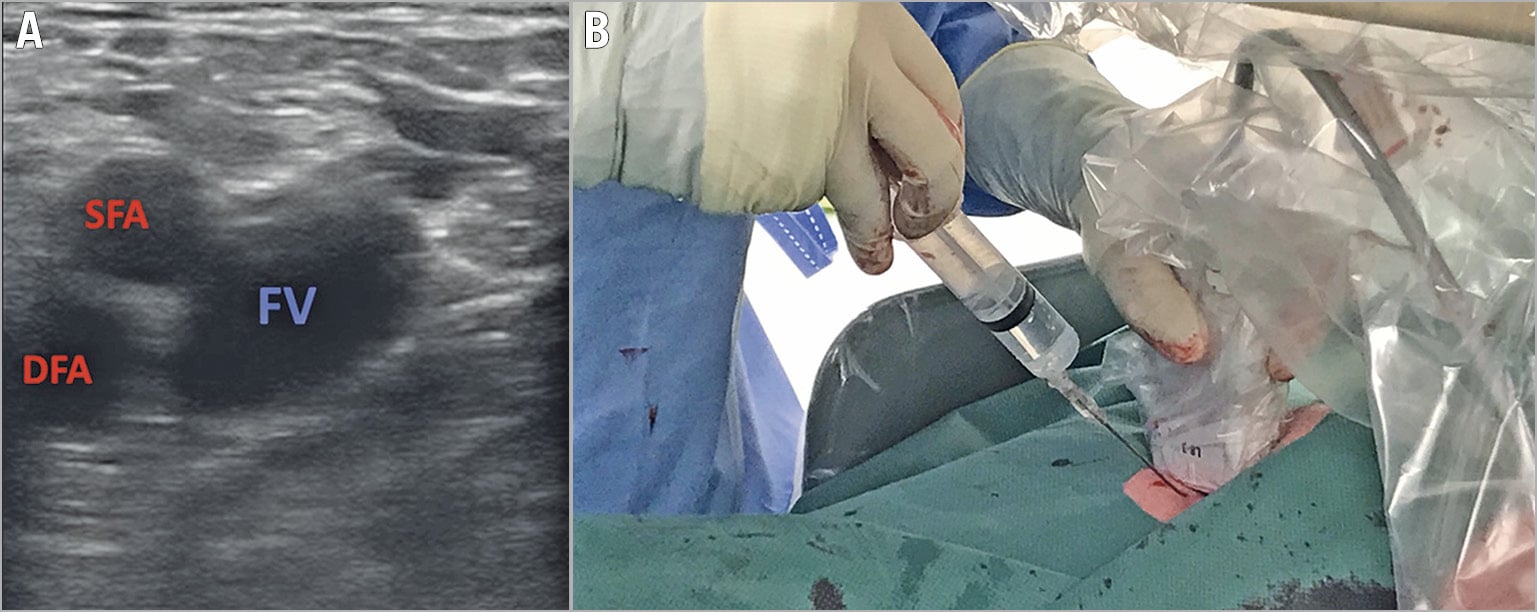
Figure 2. Echo-guided femoral punctures. A) Short-axis view. B) Operative field setting. DFA: deep femoral artery; FV: femoral vein; SFA: superficial femoral artery
During this phase, it is advisable to administer 2,000 U of heparin (~¼ of the total dose) to avoid device thrombosis because TSP may be tricky and may require additional time.
TRANSSEPTAL PUNCTURE (TSP)
1. SHEATH INSERTION
Once the femoral access has been obtained, the transseptal sheath and dilator are advanced over the guidewire to the superior vena cava (SVC) under fluoroscopy (AP projection) and TEE (bicaval view).
Tips and tricks
In case of pacemaker electrodes, the wire should be positioned posterior to them. Wire position can be checked in the left anterior oblique (LAO) projection where the wire should appear on the right side of the screen compared to the electrodes.
While advancing the sheath it is advisable to keep the sheath tip facing left (aorta) instead of right (cava free wall). In case of venous tortuosity, a larger sheath with the insertion of double stiff wires might be useful to straighten the vein and perform TSP.
2. BROCKENBROUGH NEEDLE INSERTION
The needle is advanced under fluoroscopic guidance (AP projection) until it reaches the sheath tip, avoiding pushing the needle over the tip of the sheath. The stylet prevents the needle tip from scraping the inner sheath lumen; consequently, the needle should be advanced with the stylet inside until it reaches 4 cm from the tip.
Tips and tricks
During the BRK needle insertion into the Mullins dilator, the needle should be advanced gently, allowing the arrow to rotate freely to negotiate the vascular tortuosity. After stylet removal, the Luer lock of the needle can be connected to the pressure line.
3. PULLBACK
Pullback should be started with the BRK needle arrow pointing at 5 or 6 o’clock, according to different procedures (Figure 3). To target a more posterior puncture location, the arrow should be kept at the 6 o’clock position, although IS anatomies may vary and different orientations might be needed. The needle and the sheath are pulled back caudally until they are visualised in the inferior portion of the SVC. Tenting should be monitored by echocardiography in the bicaval view and usually, as the needle tents the superior rim of the fossa, supraventricular extrasystolic beats are generated. Fluoroscopy (AP projection) helps to monitor the position (AP rotation) of the needle tip until it falls into position in the fossa and helps to avoid pulling back too much towards the inferior vena cava (IVC).
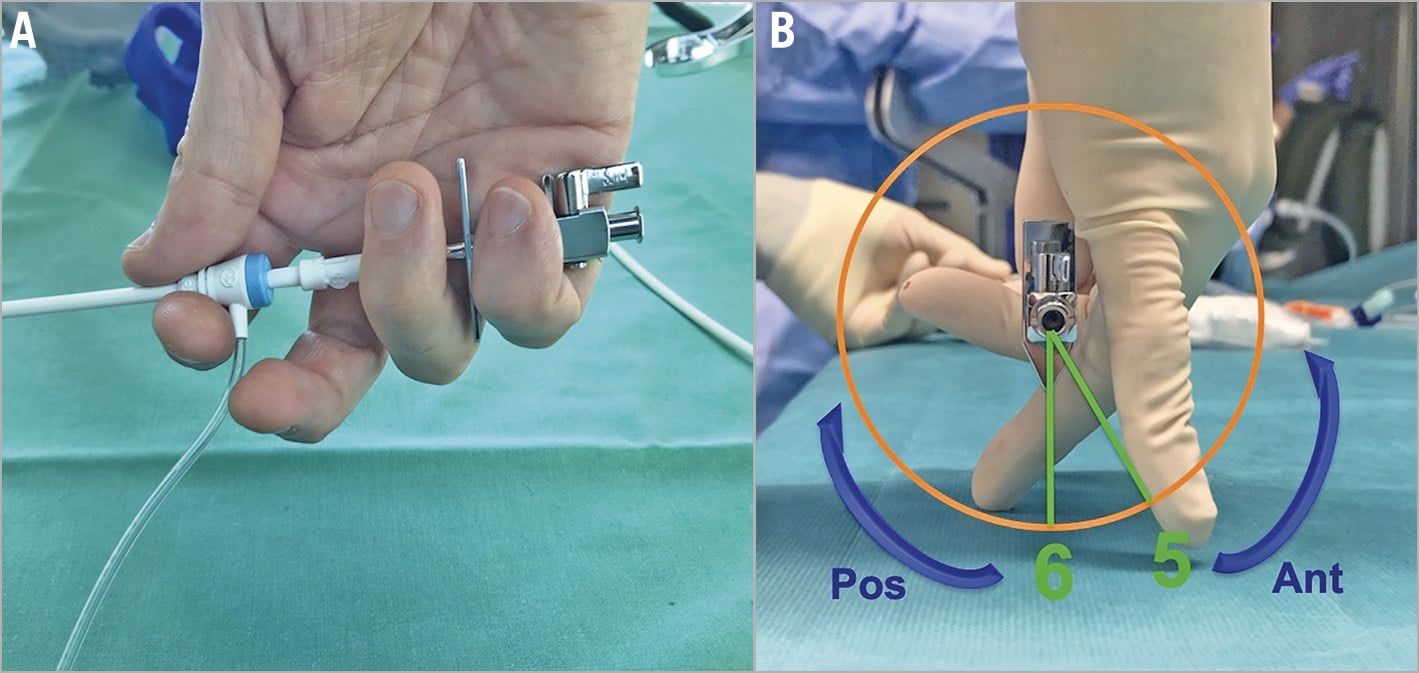
Figure 3. Correct handling (A) and orientation (B) of the Mullins/SL sheath and Brockenbrough needle during the pullback manoeuvres.
Once the sheath falls into position in the fossa and tenting is obtained (Figure 4A), TEE moves to the short axis (SAX) at the base view and position of the tenting is checked: it should be posterior in most patients undergoing mitral interventions (Figure 4B) to achieve enough working distance between the TSP site and the mitral annular level (puncture height).
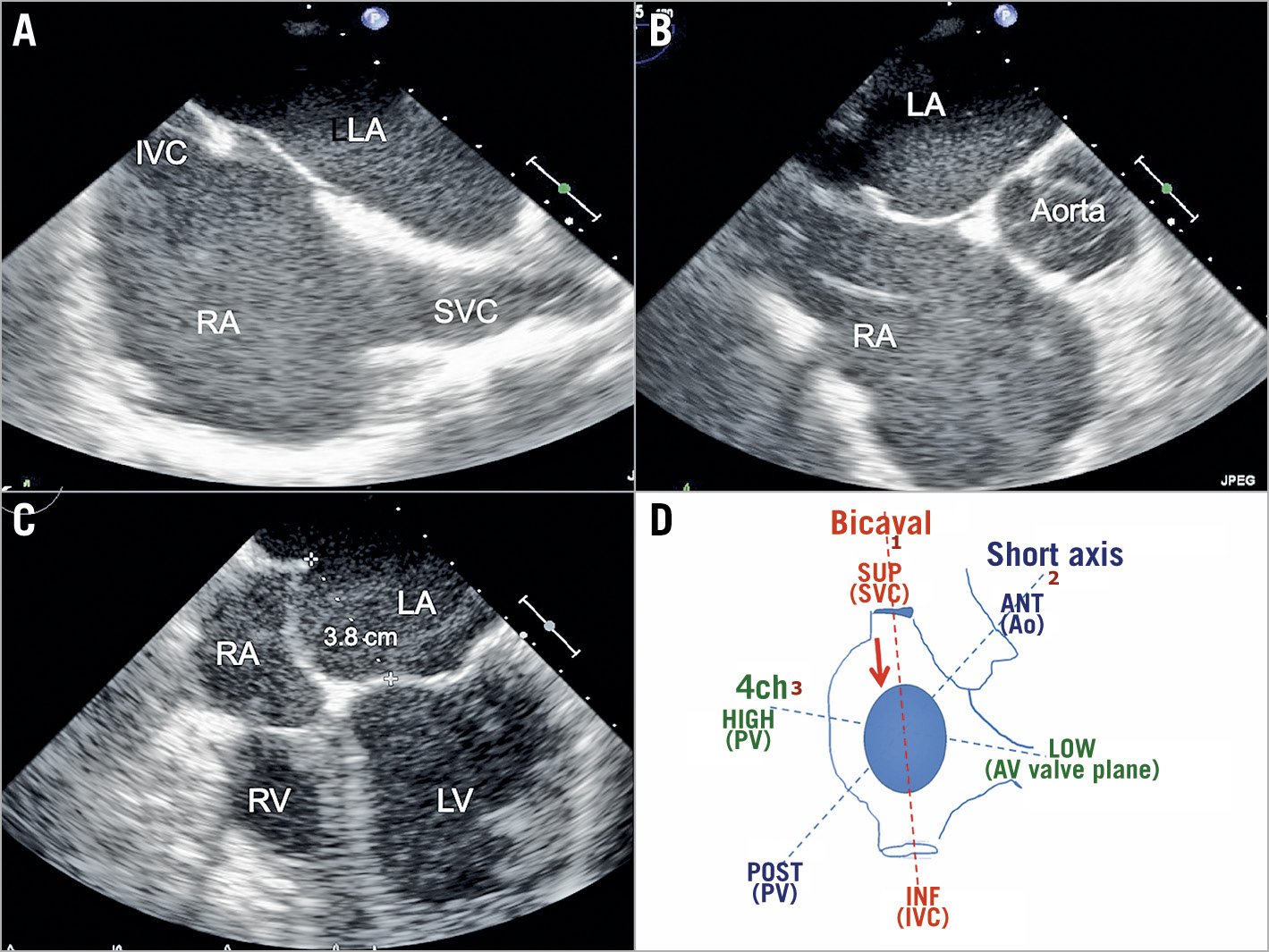
Figure 4. Views during the pullback phase of TSP. A) TEE bicaval view. B) SAX at the base. C) Four-chamber view. D) Schematic view of the three main TEE projections used during TSP in relation to the anatomic structures they show. 4ch: four chamber; ANT: anterior; Ao: aorta; AV valve: atrioventricular valve; INF: inferior; IVC: inferior vena cava; LA: left atrium; LV: left ventricle; PV: pulmonary vein; RA: right atrium; RV: right ventricle; SUP: superior; SAX: short axis; SVC: superior vena cava
In the case of MitraClip® (Abbott Vascular) procedures, TEE should now switch to the four-chamber view and measure the height of the puncture (the distance between the tenting and the mitral annulus) (Figure 4C). In patients with degenerative mitral regurgitation, the ideal height is at least 4 cm, whereas in functional mitral regurgitation a 3 cm distance is acceptable. Ideal locations for the puncture depend on the specific devices used and the patient characteristics. A MitraClip procedure is usually performed with posterior punctures in the segments superior-posterior and inferior-posterior7. A Cardioband™ (Edwards Lifesciences, Irvine, CA, USA) procedure is performed with more anterior punctures (superior-anterior and inferior-anterior), while left atrial appendage closure is usually performed through an inferior puncture (usually inferior-posterior).
The main TEE projections and related visible anatomical structures are shown in Figure 4D.
Tips and tricks
The needle and the sheath should be held as shown in Figure 3: the first three fingers hold the sheath at the level of the de-airing line, while the fourth and fifth fingers hold the needle arrow. Before starting the pullback, the de-airing line and the arrow should point in the same direction. This allows the operator to know exactly where the needle is pointing during the pullback, to rotate them concordantly and to prevent inadvertent advancement of the needle.
Once the transseptal system falls into position in the fossa, all movements to change sheath position should be in two directions at the same time to prevent needle impingement in the fossa ovalis. Indeed, an isolated clockwise rotation will result in rotation of the sheath without a change in the contact point. For instance, in case of too anterior tenting, the operator has to combine clockwise rotation with slight pulling back of the catheter in order to move posteriorly.
Clockwise rotation of the needle moves away from the mitral valve plane and allows gaining height, whereas counter-clockwise rotation moves towards the mitral valve getting closer to it.
4. TRANSSEPTAL PUNCTURE
With TEE in the SAX view and fluoroscopy in the AP projection, the needle is advanced while keeping the transseptal sheath in place. Only slow movements should be made in this phase to prevent the system from slipping into another location. Puncture of the fossa is usually followed by release of the tenting, visible on TEE. For additional safety, left atrial pressure should be confirmed before advancing the transseptal sheath. In the absence of pressure tracing or in case of aortic pressure, the sheath should not be advanced. After puncture, additional heparin to reach full heparinisation (100 U/kg) should be administered, aiming for an activated clotting time (ACT) of 250-300 seconds for most procedures.
Tips and tricks
The catheter should never be advanced before the correct position is verified by the following:
– TEE
– Blood aspiration
– Pressure curve
– Contrast injection through the needle
5. SHEATH ADVANCEMENT INTO THE LEFT ATRIUM
Both the transseptal sheath and the needle should be advanced under TEE guidance until 1 to 2 cm is in the left atrium (LA). Then, holding the needle, the sheath is advanced over the needle. Finally, the needle and the dilator are kept steady and the sheath is advanced over the dilator. When the tip of the sheath is close to the edge of the heart shadow, the needle and, eventually, the dilator can be slowly retrieved. After that, the 0.032-inch guidewire is placed into the superior left pulmonary vein with slight sheath clockwise rotation (the TEE four-chamber view is useful to confirm that the guidewire is not in the appendage). In some cases, especially when performing TMVI, septal dilatation using a 12-16 mm peripheral balloon is needed.
Tips and tricks
When using Mullins sheaths, it is advisable not to retrieve the dilator completely to avoid kinking of the sheath. Moreover, in case of very posterior puncture, it is advisable to rotate it slightly counter-clockwise before advancing in order to avoid the roof.
THE ROLE OF REAL-TIME FUSION IMAGING
A novel imaging software (EchoNavigator; Philips Healthcare, Best, the Netherlands) allows the integration between fluoroscopic and TEE images: it acquires patient-specific imaging data from both fluoroscopic projections and bi-/tri-dimensional TEE volumetric data sets and finally overlaps them. In this way, the intrinsic limitations of each imaging technique are counterbalanced by the integration of both: the optimal visualisation of cardiac valves and septa provided by TEE imaging compensates for the poor imaging details of soft tissue structures by fluoroscopy. In the same way, while fluoroscopy provides accurate images of catheters and devices, TEE gives less well-defined images due to blooming, reverberations, and shadowing.
In general, fusion imaging might simplify site-specific TSP by the addition of markers and improve eye-hand coordination as all the imaging information is displayed on a single monitor. Finally, the superimposition of fossa ovalis on fluoroscopy allows finding the correct C-arm angulation according to patient-specific ana-tomy. Possible limitations are: 1) the amount of visual information might be distracting (fusion or confusion imaging?); 2) the need for the same company’s equipment for echocardiographic machine and angiography system; 3) limited C-arm rotations which impede some overlap images for some TEE views (e.g., 3D surgical view during a MitraClip procedure); 4) once positioned, markers do not follow heart motion.
Fusion imaging has the potential to become the main imaging technique, although currently no data have demonstrated solid advantages in terms of procedural time, radiation exposure, complication rates and improvement of operator confidence.
CHALLENGING SEPTA
ENLARGED RIGHT/LEFT ATRIA
In case of enlarged atria, the TSP should be more inferior and less posterior in order to avoid exiting in the pericardial space. In some cases, if the pre-shaped curvature of the needle does not reach the IS, a further curvature to the needle by the operator may help reaching the septum.
THICK/RESISTANT SEPTUM
In case of a redo intervention after cardiac surgery or after thoracic radiation, the septum is often more resistant to cross. In such cases, alternative equipment can be used:
– The back of a 0.014-inch exchange angioplasty wire (e.g., BMW Universal; Abbott Vascular)8.
– Radiofrequency applied to the proximal part of the needle by an electrosurgical cautery generator.
– The SafeSept Needle Free (NF) Transseptal Guidewire (Pressure Products Medical Supplies Inc., San Pedro, CA, USA) has recently been tested9. SafeSept NF is a nitinol guidewire designed specifically for safer and easier transseptal puncture. The very sharp tip of the SafeSept NF easily perforates and crosses the fossa. When used in conjunction with a transseptal dilator and introducer, it creates the primary puncture in the interatrial septum, without the need for a transseptal needle. Unsupported by the dilator and sheath, the tip of the guidewire assumes a “J” shape, rendering it incapable of further tissue penetration.
– Alongside this, a technology based on radiofrequency (RF) has been developed, the Baylis radiofrequency transseptal system (NRG® RF Transseptal Kit; Baylis Medical, Montreal, QC, Canada)10. The Brockenbrough needle is substituted by an RF catheter that is introduced into the dilator/sheath assembly (TorFlex™ Transseptal Guiding Sheath; Baylis Medical). This catheter delivers 5 W energy for 2 to 5 s and can perforate the atrial septum after 1 to 4 pulses. This technology may have an advantage in thick, scarred, calcified, or patched atrial septa, where excess force could result in unsuccessful puncture or in perforation of the LA free wall: the build-up of momentum by the needle and sheath in this situation can lead to an overshoot phenomenon, in which the superior or contralateral wall of the left atrium is inadvertently punctured once the system passes through the septum.
FLOPPY SEPTUM
Given the flexibility of the membranous septum, the height of the puncture may be reduced during the steering of the devices; therefore, in the presence of a floppy septum, higher punctures may be recommended. To prevent this risk, as an alternative to conventional techniques, the puncture can be assisted by the use of a diathermy surgical system or RF transseptal system (Baylis Medical), enhancing safety and precision to the puncture11.
MANAGEMENT OF COMPLICATIONS
POSTERIOR TSP
Most mitral procedures aim at posterior TSP; while puncture of the fossa is safe, puncture of the border with the muscular septum may let the needle cross the pericardial space and cause pericardial effusion/tamponade (Figure 5A). Early diagnosis can be difficult, whereas after catheter removal at the end of the procedure it becomes clinically significant, usually causing delayed tamponade. In such cases, TEE can detect atrial haematoma (Figure 5B).
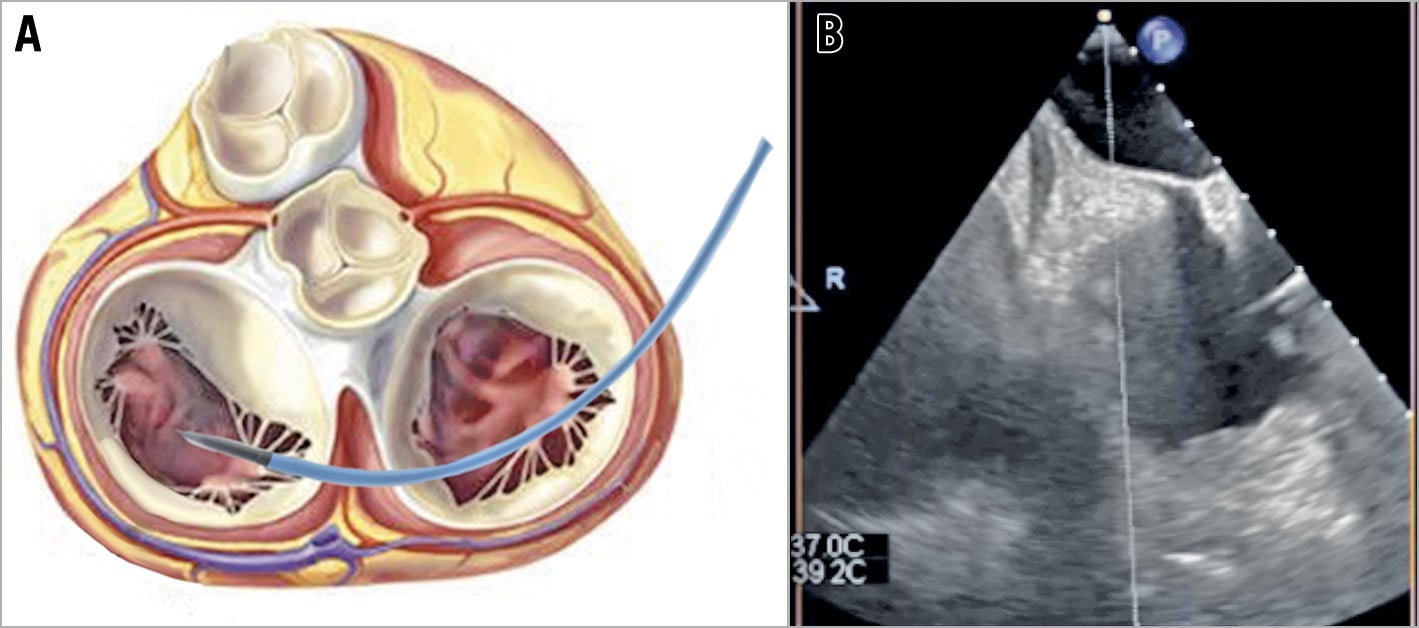
Figure 5. Posterior TSP through the pericardial space (A) causing atrial haematoma (B) and delayed cardiac tamponade.
How to manage
In case of tamponade, pericardial drainage is the first treatment. Then, bleeding breach closure through an ASD closure device is needed. Early diagnosis can be achieved by keeping the wire across the septum after removing the catheter from the LA and waiting for some minutes with arterial pressure, ECG monitoring and looking for possible pericardial effusion on TEE.
AORTA PUNCTURE
This is quite uncommon for TEE-guided TSP. However, it may occur in procedures where anterior puncture is required or due to difficult anatomy, or sometimes it might be due to insufficient experience. According to recent data, such a complication occurs in 0.05% of cases12. In case of unclear TEE images, a pressure line can be connected to the needle in order to confirm needle position. Alternatively, an injection can help to confirm needle position.
How to manage
In such cases it is important not to advance the dilator and the sheath in case of doubt about the needle position. Once the aorta puncture is confirmed, it is even more important not to pull back anything. Before planning any further steps, the surgeon should be called and, if signs of haemodynamic instability occur, cardiac tamponade is the first to be ruled out. Usually, if only the needle has been advanced, there might be no consequences, whereas, if the dilator and sheath have also been advanced, massive haemopericardium may occur. The use of occluders to fix the aorta has been described13.
EMBOLISM
Air embolism and thrombus embolism are both fairly uncommon for TSP itself. In some cases, air embolism may occur if large devices are placed across the septum and not properly de-aired. In order to avoid thrombus formation, a 1,000-2,000 IU heparin administration prior to TSP is advisable.
How to manage
In case of thrombus formation, a full dose of heparin should be administered aiming at >250 s ACT. In some cases, especially before crossing the septum, thrombus aspiration might be effective. If already in the left heart, cerebral protection placement should be considered. In cases of large thrombus formation, surgery may represent the only available solution.
ST-SEGMENT ELEVATION
This is mostly due to the Bezold-Jarisch-like reflex: ST elevation is usually located in the inferior leads and is accompanied by vagal signs (hypotension, bradycardia and diaphoresis). It is probably caused by the high density of parasympathetic fibres in the septum leading to cholinergic vasospasm.
How to manage
Recovery without troponin rise occurs after a few minutes and atropine administration is seldom necessary. Alternatively, if ST elevation persists, air or thrombus embolism might be the cause and should be treated accordingly.
IATROGENIC ATRIAL SEPTAL DEFECT
This is a common finding after large-bore sheath transseptal access. The strongest predictor for the development of persistent ASD is the size of the sheath or guiding catheter14, while the role of left atrial pressure is more controversial. The most frequent indications for ASD closure are bidirectional or right-to-left shunt and large ASD (>8 mm). Consequently, it is highly advisable after TMVI procedures, as very large bore sheaths and septal balloon dilatation are required.
How to manage
When any of the above-mentioned situations occur, immediate ASD closure is advisable. In case of conservative treatment, special attention should be reserved for those patients with right-to-left shunt and risk factors for developing deep venous thrombosis. In such cases, empiric anticoagulation might be needed to avoid paradoxical embolism, stroke or systemic embolisation.
Conclusions
Although the technique and equipment have not undergone huge changes since the first description in the late 1950s, the growing use of TSP and the need for precise and safe puncture have brought new devices and improved the technique. The most important adjunctive feature has been intraprocedural echocardiography (TEE or intracardiac echo) because it adds precision and safety and also allows less experienced operators to perform TSP. The combination of fluoroscopic and transoesophageal echocardiographic images may further facilitate the procedure15. However, some complications or challenging situations might occur. For this reason, an in-depth knowledge of all procedural steps and possible complications is important as well as supervision by more experienced operators during the initial phase of the learning curve. Alongside technical skills, TSP training should also include echocardiography and cardiac tamponade management.
Conflict of interest statement
F. Maisano is a consultant for Abbott Vascular, Medtronic, Edwards Lifesciences, Perifect, Xeltis, Transseptal Solutions, Magenta and Cardiovalve, has received grant support from Abbott Vascular, Medtronic, Edwards Lifesciences, Biotronik, Boston Scientific, NVT, and Terumo, has royalty income/IP rights from Edwards Lifesciences and 4Tech, and is co-founder/shareholder of Transseptal Solutions, 4Tech, Cardiovalve, Magenta, Perifect, Coregard and SwissVortex. G. Russo has received a fellowship training grant from the EAPCI, sponsored by Edwards Lifesciences. M. Taramasso reports consultancy fees from Abbott Vascular, Edwards Lifesciences, 4Tech, Boston Scientific, CoreMedic, Mitraltech, and SwissVortex, outside the submitted work.
Supplementary data
To read the full content of this article, please download the PDF.

