Abstract
Percutaneous transapical access provides a direct route to many cardiac structures difficult to reach with conventional interventional approaches. With recent developments of new technologies in structural heart disease, there has been an increasing interest in the use of transapical access for cardiac interventions. Meticulous planning, careful access and closure techniques are essential. Development of novel imaging technologies and dedicated closure devices are warranted to allow a greater number of operators to successfully adopt percutaneous transapical access and further reduce complication rates. This article is an overview of the current status and utility of percutaneous transapical access with focus on multimodality imaging, technique and potential complications of this approach.
Introduction
For more than half a century, transapical left ventricular access has been used for diagnostics and haemodynamic assessment1-3. However, recently, with the development of new technologies in structural heart disease, there has been an increasing interest in the use of transapical access for cardiac interventions. Currently, transapical access is mostly being used for transcatheter aortic valve replacement (TAVR), with the approach performed exclusively via an open surgical mini-thoracotomy. Our group pioneered the technique for the percutaneous approach to the left ventricle, applied to a multitude of structural interventions including the closure of paravalvular leaks, left ventricular (LV) pseudoaneurysms, complex ventricular septal defects and the implantation of transcatheter mitral valve-in-valve4-10. Transapical access can facilitate navigation within the left ventricle in a more precise and accurate manner, and ultimately can lead to a decrease in fluoroscopy and procedural time in comparison to the transaortic or the transseptal approach7,10. The purpose of this article is to give an overview of the current status and utility of percutaneous transapical access with a focus on multimodality imaging, technique and potential complications of this approach.
Patient selection
Providing direct access to the LV transapical approach can be used for LV targets at locations that are difficult to reach with the antegrade transseptal or retrograde transaortic approach due to unfavourable angles, extensive calcification surrounding the target lesion or prominent papillary muscles. It can also be used as an alternative approach in patients with severe peripheral atherosclerotic disease or for creation of a delivery rail (transseptal-transapical, aorto-transpical) to provide additional support for the delivery system and to allow precise deployment of the device(s).
The majority of the percutaneous transapical access experience has been in patients with previous cardiac surgery requiring pericardiotomy. These patients are less likely, but not immune, to develop haemopericardium and tamponade because of the presence of pericardial adhesions. In the early post-operative period, the risk of bleeding remains high. For these reasons, our preference is to wait at least three months after the initial surgical pericardiotomy. Percutaneous transapical access should be avoided in patients without prior cardiac surgeries, as they are considered, at the present time and with the currently available technology, to be at extreme risk of bleeding and tamponade.
Absolute contraindications for the transapical access are a fresh clot within the left ventricle and a large apical left ventricular aneurysm. We previously reported death from electromechanical dissociation without evidence of tamponade in a patient with suprasystemic pulmonary hypertension; hence, we consider this group of patients to have significant risk for periprocedural mortality7. Furthermore, haemodynamically significant aortic stenosis left untreated, hypocoagulable states or any other bleeding diathesis pose additional procedural risks.
Imaging: procedural planning and guidance
FLUOROSCOPY AND ECHOCARDIOGRAPHY
In early reports, transapical access for diagnostic purposes was performed without imaging guidance, using just anatomical references and pressure recordings1-3. Since then, several imaging modalities have been used for the guidance. Most involve a combination of fluoroscopy and echocardiography4,11-13. Fluoroscopy with coronary angiography can be useful in defining the coronary anatomy during puncture, avoiding injury to the left anterior descending artery (LAD) (Figure 1).
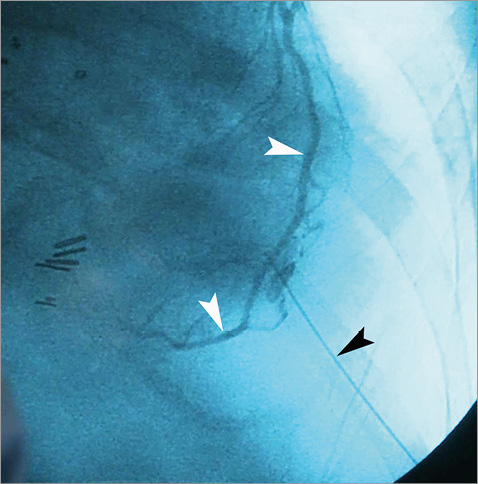
Figure 1. Selective coronary angiography during transapical puncture. Left descending artery (white arrowheads) can be identified during insertion of the 21G micropuncture needle (black arrowhead).
Transthoracic echocardiography can help with the delineation of the LV apex, determination of angle for puncture and, at the same time, monitoring for immediate complications such as haemopericardium or haemothorax. Areas of myocardial thinning, scarring or focal wall abnormalities, along with presence of LV apical thrombus can be evaluated. These modalities, however, are limited in demonstrating the complex three-dimensional relationship between the left ventricle, chest wall and needle. Transoesophageal echocardiography (TEE) can be used during the procedure for the monitoring of complications and during device closure, but its utility is restricted (Figure 2).
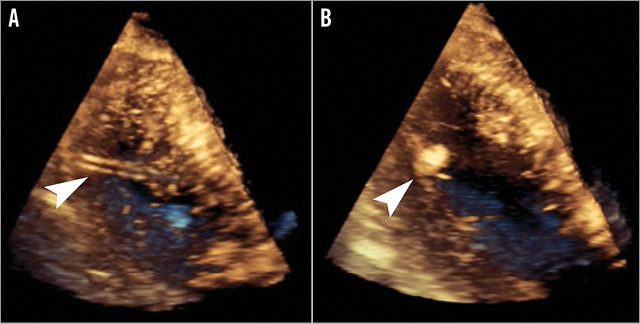
Figure 2. Three-dimensional transoesophageal echocardiography during transapical access closure. Visualised within the left ventricle are the delivery sheath (A) and an Amplatzer Vascular Plug II (B) with the distal disc being positioned against the endocardial surface.
COMPUTED TOMOGRAPHY AND FUSION IMAGING
Computed tomographic angiography (CTA) can be very useful in the planning of transapical access. With 3D volume-rendered reconstruction, regional anatomy can be easily delineated, including extension of the lung tissue over the LV cavity, location of the cardiac apex and coronary arteries, and even the anatomy of the papillary muscles (Figure 3). The distance from the skin to the LV apex, optimal intercostal space and appropriate angle for entry can all be obtained from the CTA (Figure 4)14.
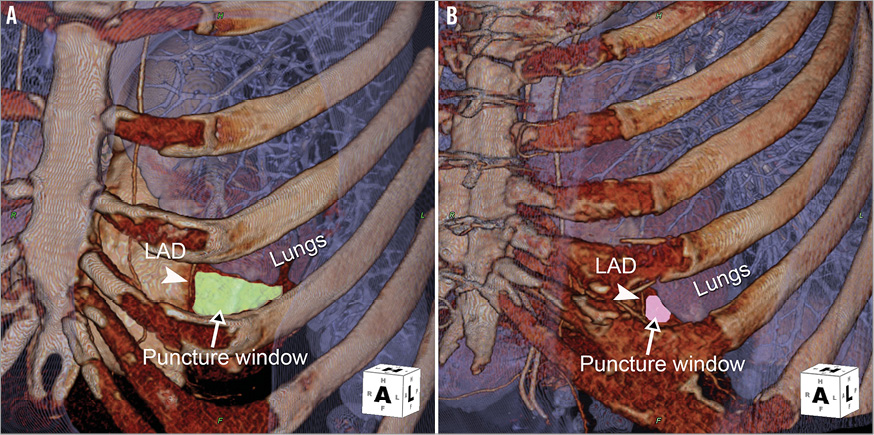
Figure 3. Three-dimensional, volume-rendered computed tomographic angiography (CTA) demonstrates variations of the “safe puncture window” in different patients. A) Patient with good exposure of the LV apex with a large puncture window. B) Patient with small puncture window because of lung overlap and close proximity of the coronary artery.
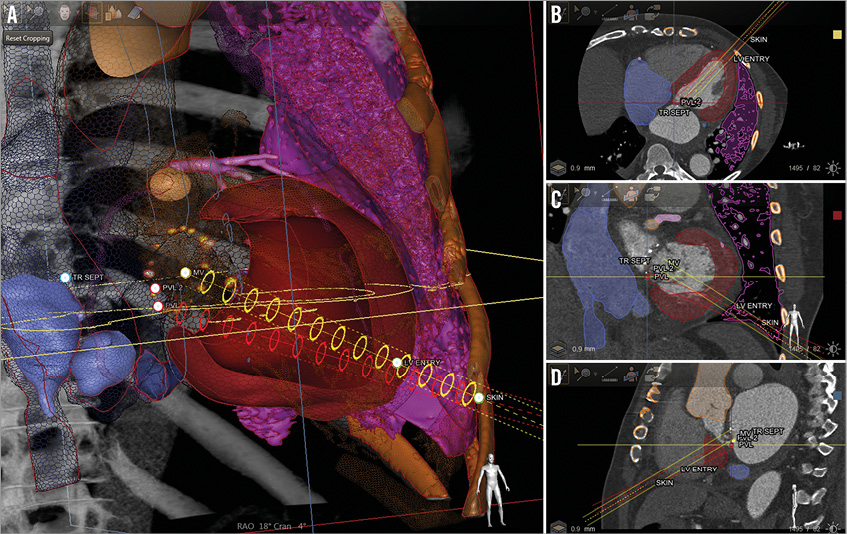
Figure 4. Planning of the transapical access with model-based automatic cardiac segmentation using computed tomographic angiography (CTA) data (HeartNavigator; Philips Healthcare, Best, The Netherlands). A) Three-dimensional model of the heart with white landmarks placed on the location of skin entry, left ventricular epicardial entry, and target structural heart defect – paravalvular leaks. Safe paths (red and yellow cylinders) are generated within the 3D model between the ribs connecting the landmarks, avoiding the coronary arteries and lung. B, C and D) Axial, coronal and sagittal standard CT planes with the projection of safe paths are noted. The position of the landmarks and their respective paths can be adjusted manually on any of these planes if needed. The 3D model is subsequently overlaid onto live fluoroscopy in the catheterisation laboratory.
Furthermore, we use the CTA for CT-fluoroscopy fusion imaging (HeartNavigator; Philips Healthcare, Best, The Netherlands) to guide the procedure and, in our experience, it plays a considerable role in safe percutaneous transapical access. The 3D volume-rendered CTA is first segmented, identifying important cardiac structures, ribs and the lungs. Then, preselected landmarks are placed to identify the skin entry, left ventricle epicardial entry and the structural heart target that requires the intervention (Figure 5). The CTA is subsequently registered with fluoroscopy, and outlines of these structures with the landmarks are overlaid onto the x-ray image. With this technique we are able to achieve puncture accuracy within 5 mm of intended entry and approximately 15 mm away from the LAD14. If fusion imaging software is unavailable, CTA images in equivalent angulations can be projected on a monitor adjacent to the fluoroscopic image to help guide the ventricular access5,7. The latter is less reliable, and heavily operator-dependent.
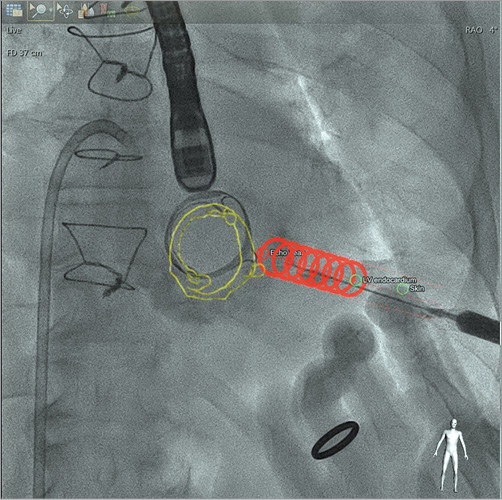
Figure 5. Computed tomography-fluoroscopy fusion imaging. Live fluoroscopy is presented during transapical puncture with CT overlaid cardiac structures (prosthetic valve - green outline), landmarks (skin and epicardial entry - yellow dots), and “safe path” (red circle cylinder) identified.
Technique of percutaneous left ventricular access
All procedures are performed in hybrid operating rooms or catheterisation laboratories under general anaesthesia with a surgical team on standby. If there is concern for lung extension over the desired puncture site, lungs can be temporarily deflated with breath-hold or selective lung ventilation using a double-lumen tube or endobronchial balloon. Radiolucent defibrillator pads should be placed on the chest and back, so as not to obscure cardiac fluoroscopic views. The patient should be prepped and draped in a sterile fashion from the clavicles to the groin, in case emergent thoracotomy is needed.
Once the transapical puncture site has been selected, the skin is entered with a 21 gauge micropuncture needle guided toward the site of LV epicardial entry. In most cases, the LV can be reached with a 7 cm needle, but sometimes a longer 15 cm Chiba or even 20 cm spinal needle may be needed. After cannulation of the LV is confirmed with contrast injection, a 0.018 inch guidewire is advanced into the left ventricle, then into the left atrium or the aorta, with the position of the wire confirmed by fluoroscopy and TEE. The needle is exchanged for an appropriately sized delivery sheath, typically braided for improved support, according to interventional needs, with reported sizes ranging from 5 Fr to 12 Fr. The size of the sheath should be determined in advance, to minimise the need for multiple sheath exchanges.
Occasionally, more than one access to the LV is needed. We have experience with double and triple access for simultaneous deployment of several devices for paravalvular leak closure without any adverse events. It is important, however, to maintain a safe distance between transapical entry points, such that distinct punctures are available for closure without interference between closure devices. Nonetheless, we would recommend avoiding multiple LV accesses when possible due to limited long-term data.
Techniques for closure of the percutaneous LV access
We previously reported our experience with transapical access closure7,14. In four patients, utilising transapical access sheaths of 5 Fr, the access site was not closed. No complications were noted in this group; however, our practice is to routinely close all puncture sites. Currently, there are no FDA-approved devices for apical closure, but several devices have been utilised “off-label”. We have used devices of the Amplatzer family (St. Jude Medical, St. Paul, MN, USA) –muscular ventricular septal defect (mVSD), Amplatzer ductal occluder (ADO) and Amplatzer vascular plug II (AVP II) devices (Figure 6A-Figure 6C). Presently, our most commonly used device is the AVP II, which we believe is the most suitable device for this purpose until specifically designed devices are available.
Prior to deployment of the closure device anticoagulation is reversed with protamine. The implantation of the closure device is performed under real-time fluoroscopy and TEE guidance. If fluoroscopy is used, the small amount of contrast injection through the sheath during the withdrawal will identify when the sheath approaches the myocardium/pericardial space, with the disc of the device secured against the endocardial surface. At this moment, elongation and systolic compression of the device should be noted through the cardiac cycle to confirm location. With 3D TEE, the operator can follow the device during withdrawal to allow for precise positioning.
Presently, novel open surgical transapical closure devices that replace the classic purse-string suture technique are in development. Three technologies are now “first-in-man” and include the application of conical-shaped coils which employ radial compression with a sealing cap (Apica; Thoratec, Pleasanton, CA, USA), helical needles that drive sutures into the myocardium with endocardial anchors and epicardial locking buttons (CardioClose; Entourage Medical, Menlo Park, CA, USA) and transmyocardial anchors with elastomeric V-stays that expand and contract creating an operative window (Permaseal; MicroInterventional Devices Inc, Newtown, PA, USA) (Figure 6D-Figure 6F). Potential percutaneous iterations of these technologies may provide even more effective closure.
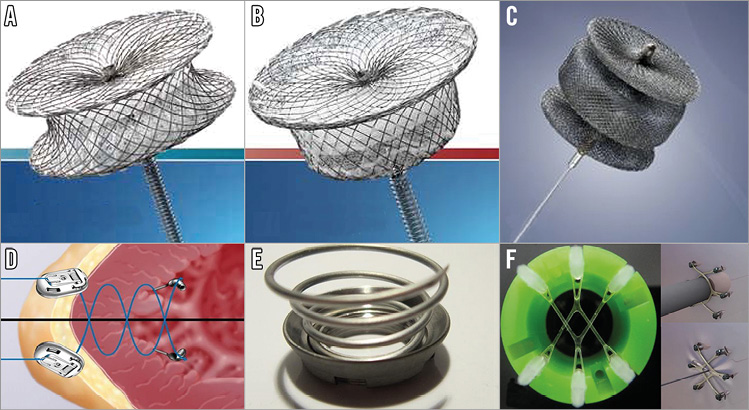
Figure 6. Devices used for completely percutaneous transapical access closure. AMPLATZER family devices: A) Muscular Ventricular Septal Defect Occluder; B) Duct Occluder; C) Vascular Plug II. Systems for closure of surgically exposed transapical access: D) CardioClose; E) Apica ASC; F) Permaseal.
Outcomes
This technique is not without complications, such as haemothorax, pericardial effusion and tamponade, coronary laceration, pneumothorax, cardiac arrhythmia and death. Historically, complication rates for transapical access ranged between 3 and 10%3,13; however, this was for diagnostic procedures solely with needle puncture. With the use of transapical access for structural interventions, the complication rate can be much higher4. Pitta et al reported their experience with 32 patients: 19 patients underwent diagnostic procedures and 13 patients underwent structural interventions4. Complications were observed in four patients (21%) in the diagnostic group and eight patients (62%) in the interventional group. The access technique was similar in both groups, therefore attributing the difference to the fact of leaving the access site without closure.
Our group reported experience with 28 patients who underwent 32 transapical punctures. There was one instance of a small pericardial effusion documented by echocardiography that required no further intervention, and one procedure-related death in a patient with suprasystemic pulmonary hypertension who developed pulseless electrical activity (electromechanical dissociation) immediately after the procedure. Two-dimensional echocardiography and emergency thoracotomy did not show any pericardial effusion. In our more recent experiences, additional complications that we have encountered include pneumothorax, cardiac tamponade, closure device embolisation and LV pseudoaneurysm formation (Figure 7).
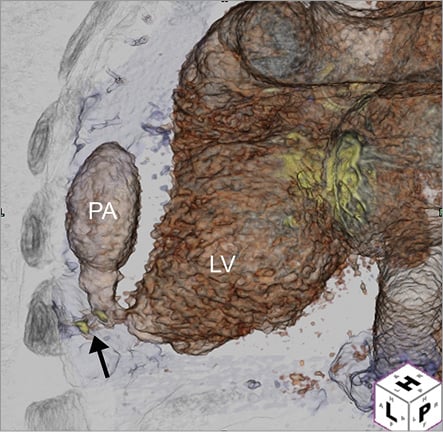
Figure 7. Three-dimensional reconstruction of post-procedural computed tomographic angiography demonstrated a large left ventricular (LV) pseudoaneurysm (PA, black arrow) at the transapical puncture site.
Overall, haemothorax is the most frequent complication associated with this technique. It can be related to coronary or intercostal vessel laceration or bleeding from the LV puncture site. Coronary laceration can potentially be avoided with CT imaging guidance and coronary angiography when obtaining transapical access7,14. A chest tube may be required for evacuation of the haematoma if the patient is symptomatic or difficult to ventilate. For cardiac tamponade, pericardial drainage is required with percutaneous drainage generally sufficient. However, emergent left lateral thoracotomy may be required to control access-site bleeding, with intramyocardial placement of pledgeted sutures.
Conclusion
Percutaneous transapical access is an invaluable approach for many cardiac structural interventions, providing direct entry into the LV with the potential of increasing procedural success rates7,10. Careful procedural planning should be made utilising CTA, echocardiography and fluoroscopy. The implementation of new imaging technology such as CT-fluoroscopy fusion imaging provides a more accurate, and potentially a safer, means for access.
Equally important to LV entry is transapical closure. Many closure devices including several of the Amplatzer family of devices have been used for percutaneous transapical closure with good results. However, more long-term follow-up of this approach is needed. Novel percutaneous closure devices are being investigated which may further reduce complication rates and may broaden the range of sheath sizes and applications of this technique.
Conflict of interest statement
C. Ruiz receives fees from ValTech, St. Jude Medical & Sorin for consulting, receives ownership interest from Vascular Therapies, MitrAssist and Entourage for partnership, and receives research grants from Philips. C. Kliger receives an honorarium from St. Jude Medical and Philips for speaking. I. Kronzon receives consultant fees from St. Jude Medical and honoraria from Philips Healthcare. The other authors have no conflicts of interest to declare.

