
Abstract
Aims: Percutaneous structural heart therapies, such as mitral value repair, require site-specific transseptal access (TSA). This can be challenging for interventional cardiologists. We describe a TSA catheter (TSAC) that utilises suction for enhanced control and puncture accuracy. Here, we aim to evaluate the safety and efficacy of the device.
Methods and results: Ex vivo interatrial septum preparations were dissected from swine (n=8) and diseased human hearts (n=6) to quantify TSAC suction and needle puncture force. TSAC suction was 6.5-fold greater than the opposing needle puncture force, and thus provides sufficient stabilisation for punctures. The safety and efficacy of TSAC was evaluated in a chronic mitral regurgitation swine model (n=10) and compared to a conventional TSA device. MR was induced by disrupting one to three mitral chordae tendineae, and the progression of heart disease was followed for three weeks. During device testing, procedure time and fluoroscopy exposure were not statistically different between devices. TSAC reduced septal displacement from 8.7±0.30 mm to 3.60±0.19 mm (p<0.05) and improved puncture accuracy 1.75-fold.
Conclusions: TSAC provides controlled TSA and improves puncture accuracy, while maintaining procedure time and workflow. These findings provide a strong rationale for a first-in-man study to demonstrate the clinical utility of the device.
Introduction
Transseptal access (TSA) to the left atrium (LA) was initially introduced in 1959 for LA pressure measurements1,2, and is currently an important and rapidly growing procedure for diagnostics and therapy delivery3-9. Emerging percutaneous approaches for structural heart therapies require a highly specific TSA site across the fossa ovalis (FO). For example, mitral valve repair with the MitraClip® (Abbott Vascular, Santa Clara, CA, USA) calls for a superior-posterior transseptal (TS) puncture to align the clip properly above the centre of the mitral valve orifice and the origin of the regurgitant jet8. Early clinical experience with the MitraClip has revealed that suboptimal TSA requires the device to be removed and TSA re-established leading to greater procedure duration and X-ray exposure8,10-12. In a different application, left atrial appendage closure relies on an inferior-posterior puncture to implant devices successfully13. Even with imaging modalities such as intracardiac echocardiography and transoesophageal echocardiography (TEE), the continuous motion of the heart and the evasive nature of the FO can make accurate punctures difficult.
Accurate TSA is complicated by the progression of structural heart disease that remodels the interatrial septum. In patients with FO aneurysms, the septal tissue is so pliable that, during TS needle advancement, the tissue distends to the lateral or posterior wall of the LA and cardiac perforation must be avoided14-17. Conversely, septal remodelling and/or multiple TS punctures over several procedures may lead to a thick or fibrous FO that is resistant to a conventional TS puncture. Resistant TSA occurs with 5% of punctures18,19, and is expected to increase in frequency due to the large number of electrophysiology and interventional cardiology procedures using TSA6,11,12,20.
Transmission of radiofrequency energy has been shown to provide a safe method for piercing the FO when conventional approaches fail18,21. Radiofrequency approaches reduce the mechanical force on the FO; however, like traditional needle punctures, they cannot easily control the location of the TS puncture.
Here, we describe a low-profile TSA catheter (TSAC) that utilises suction to grasp and hence immobilise the FO for controlled and accurate TSA. The stabilising force produced by the TSAC as well as the opposing TS puncture force were quantified on ex vivo swine and human heart preparations. The safety and efficacy of the TSAC device were tested in vivo on domestic swine with chronic mitral regurgitation (MR) and compared against a conventional TS device (CTD).
Methods
TRANSSEPTAL ACCESS DEVICE
TSAC has been designed specifically to improve the safety and accuracy of TS punctures by stabilising the FO by a reversible suction mechanism. The design features of the TSAC include: 1) a double lumen sheath that separates suction from the needle-dilator assembly, 2) an 8 mm bellowed cup, and 3) an outer peel-away introducer. Covering the double lumen sheath is a peel-away introducer that mechanically collapses the cup for insertion into the femoral vein. The double lumen sheath is a 12 Fr fixed curve with a suction lumen that is patent under wall vacuum (300 mmHg) and a dilator-needle lumen that provides access to the FO. The bellowed suction cup is atraumatic and composed of medical grade silicone, which is easily collapsed by the outer peel-away introducer but is strong enough to remain patent during suction.
TSAC integrates well with TSA tools (i.e., 0.035” guidewire, dilator, and needle) and imaging modalities. As with the conventional approach, a dilator and guidewire are placed within the TSAC and the unit is inserted into the femoral vein with the cup collapsed. The system is advanced to the superior vena cava (SVC) using fluoroscopy, and then the cup is released and the guidewire is exchanged for a TS needle. With the needle safely inside the dilator, a drag-and-drop technique is used to position the device in the right atrium (RA) near the FO. The cup gently presses against the FO, and wall vacuum is subsequently turned on to secure the tissue inside the cup. Firm attachment to the FO is confirmed by TEE or fluoroscopy. If the location is determined to be unsatisfactory, the vacuum is released and the position of the cup is adjusted. Once the cup is spatially positioned in an ideal location, the TS needle is controllably advanced into the FO.
HEART STUDIES
Domestic swine and explanted human hearts were used to evaluate TSAC. All animal protocols were approved by the Institutional Animal Care Use Committee at the California Medical Innovations Institute (San Diego, CA, USA). Human subjects gave informed consent, and donated hearts were provided by Save A Life America (San Diego, CA, USA).
EX VIVO BENCH TESTING
For TSAC to maintain attachment to the FO during the puncture, the stabilising suction force (FS) must be greater than the needle puncture force (FP). Forces were evaluated on FO dissected from healthy swine hearts (n=8) and human hearts (n=6) rejected for transplantation.
Swine were placed under deep anaesthesia with 5% isofluorane, then saturated potassium chloride (120 ml) was injected intravenously to arrest hearts. Swine hearts were quickly explanted with a midsternal incision and washed with Tyrode’s solution (128.2 NaCl, 1.3 CaCl2, 4.7 KCl, 1.05 MgCl2, 1.19 NaH2PO4, 20 NaHCO3 mmol/L).
Post-mortem human hearts were procured within four hours of death. Hearts were submerged in cold saline (~4°C) during transport to our laboratory. Human hearts were from male (n=1) and female donors (n=5) with an average age of 84 years. Causes of death included congestive heart failure (n=1), myocardial infarction (n=3), renal failure (n=1), and lung cancer (n=1).
The FO was carefully dissected and prepared for one of the following three force measurement experiments: 1) TSAC suction force, 2) needle puncture force, or 3) simultaneous suction-puncture force. For all force experiments, the preparation was securely attached to a force transducer (ADInstruments Pty Ltd., Bella Vista, NSW, Australia) and immersed in room temperature saline solution. Force measurements as well as the wall vacuum pressure were recorded with PowerLab® 16/35 (ADInstruments). The maximum Fs was determined by grasping the tissue with the TSAC connected to wall vacuum and then withdrawing the TSAC until the device detached. Fs was considered the force at which the bellowed suction cup detached. Fp was quantified by advancing the Heartspan™ transseptal needle (Boston Scientific, Marlborough, MA, USA) through the FO. Simultaneous suction-puncture force testing was performed similar to in vivo workflow. First, vacuum was initiated resulting in the TSAC securing tissue, and then the device was lightly pulled back approximately 1 N as the Heartspan needle was advanced through the FO.
IN VIVO ANIMAL TESTING
The safety and efficacy of TS punctures with the TSAC was evaluated in a chronic MR swine model. A survival surgery was first completed to induce MR followed by a terminal surgery to test a TSAC (n=5) and a CTD (n=5).
Swine of either sex (40-50 kg) were initially sedated with an intramuscular injection of a Telazol (2 mg/kg), ketamine (1 mg/kg), and xylazine (1 mg/kg) cocktail. Animals were ventilated via a mechanical respirator and general anaesthesia was maintained via 1-2% isofluorane with 100% oxygen. Baseline two-dimensional transthoracic echocardiographic (TTE) images were recorded (S5-1, Philips iE33 Ultrasound System; Philips Healthcare, Andover, MA, USA) using apical and subcostal windows to evaluate structural, functional, and haemodynamic parameters. The jugular vein was accessed for the placement of a Swan-Ganz™ catheter (Edwards Lifesciences, Irvine, CA, USA), which was positioned in the pulmonary artery for real-time assessment of pulmonary artery wedge pressure. A TEE probe (X7-2t; Philips Healthcare) was placed to visualise the mitral valve and the degree of MR.
MR was induced by disrupting one to three mitral chordae tendineae22,23. A long 7 Fr sheath (Flexor® Ansel Guiding Sheath; Cook Medical, Bloomington, IN, USA) was inserted into the left ventricle (LV) through the carotid artery. Under fluoroscopy guidance, an avulsion wire (custom-made, Cook Medical) was advanced through the sheath and chordae tendineae were severed. After inducing MR, swine were recovered and followed for three weeks with weekly TTE imaging.
Using a standard clinical approach17,24, TS punctures were completed with either a CTD (HeartSpan® Braided Transseptal Sheath; Merit Medical Systems, Inc., South Jordan, UT, USA) or a TSAC. For both TS devices, TEE and fluoroscopy were used to verify a safe needle position prior to TS puncture and movies were recorded continuously throughout the TS puncture. LA access was confirmed with both contrast injection and an LA pressure recording. After completion of the TSA procedure, hearts were explanted and the interatrial septum was dissected to assess puncture accuracy and inadvertent tissue damage.
DATA ANALYSIS
The safety and efficacy of the TSAC was assessed for the following: 1) procedure complications, 2) procedure time, 3) fluoroscopy time, 4) total fluoroscopic energy emitted, 5) interatrial septum displacement and tenting during puncture, and 6) puncture accuracy. The procedure time, fluoroscopy time, and total fluoroscopic energy emitted were calculated from the start of the drag-and-drop to confirmation of LA access. Interatrial septum displacement and curvature were determined using TEE recordings taken during TS puncture. Curvature (k) is defined as the inverse of the radius (r) of a circle or k=1/r. Tissue curvature was quantified by fitting a circle to the interatrial septum in TEE images at the point of TS puncture (MATLAB® Image Processing Toolbox™; MathWorks, Natick, MA, USA).
Puncture accuracy was determined from photographs of dissected interatrial septa, where the centroid of the FO was the target. The FO was delineated manually and the centroid of the FO was computed using MATLAB software (Image Processing Toolbox; MathWorks). The distance between the centroid and the puncture site was normalised to half the length of the major axis of the FO to negate differences in FO geometry.
STATISTICAL ANALYSIS
All data are expressed as the mean±standard error of the mean. A p-value of 0.05 was considered significant. Statistically significant differences were identified using a Student’s t-test or a one-way analysis of variance followed by a post hoc Tukey test.
Results
STABILISING SUCTION AND PUNCTURE FORCE
Figure 1A provides an illustration of a TSAC engaged with the FO and shows Fs (purple arrow) and Fp (teal arrow) on the tissue. When wall vacuum (300 mmHg) was connected to the TSAC, the maximum Fs that was produced by the TSAC was –7.73±0.12 N (Figure 1B). The opposing TS needle Fp on the FO was quantified in both healthy swine (n=8) and diseased human hearts (n=6), and was found to be 0.63±0.05 N and 1.17±0.18 N, respectively. The puncture force measured in diseased human FO was statistically greater than in healthy swine FO (p=0.002). Overall, these data highlight that the stabilising suction force produced by the TSAC is sufficient to withstand the opposing TS needle puncture (6.5-fold greater) and maintain secure FO attachment.
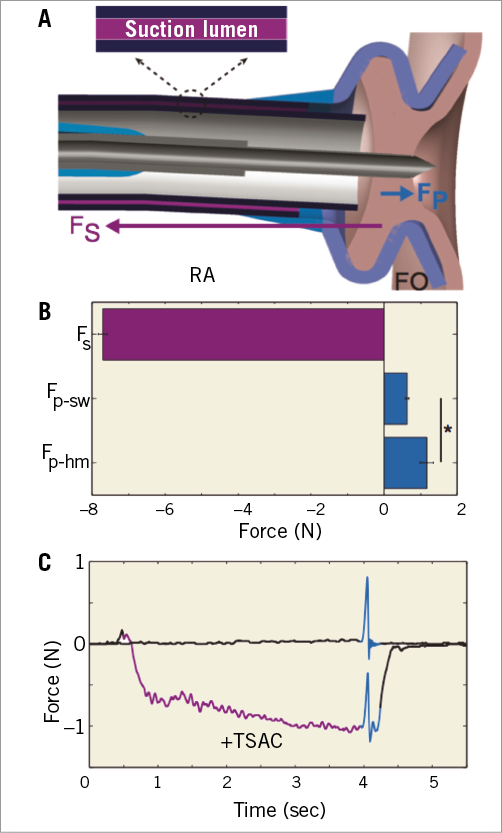
Figure 1. Stabilising suction force (Fs) provided by the transseptal access catheter (TSAC) and the puncture force (Fp) of the transseptal needle. A) Illustration of TSAC engaging the fossa ovalis (FO) and arrows representing the magnitude and direction of Fs and Fp. B) The magnitude of Fs (n=8) is greater than Fp of swine (Fp-sw, n=8) and Fp of humans (Fp-hm, n=6). C) Representative tracing of FO puncture with application of a gentle –1.0 N tissue deflection prior to puncture (+TSAC) and FO puncture without TSAC. Suction application - purple, FO puncture - teal. *p=0.002. FO: fossa ovalis; Fp: needle puncture force; Fp-hm: needle puncture force in human; Fs: suction force; RA: right atrium; TSAC: transseptal access catheter
Figure 1C displays representative force tracings of a TS puncture without TSAC (top) and with TSAC (bottom). When the TSAC was utilised, a small force (-1.0 N, purple) was applied to the FO, displacing the tissue in the opposite direction of the upcoming TS puncture. As the TS needle was advanced through the displaced FO, there was an increase in force (teal), and the summation of Fs and Fp was less than 0 N. The data indicate that the TSAC can manipulate the FO to counterbalance Fp, which would diminish interatrial displacement by the TS needle and decrease the likelihood of cardiac perforations in the posterior and lateral LA.
CHRONIC MITRAL REGURGITATION SWINE MODEL
The safety and efficacy of TS punctures with the TSAC was evaluated in a chronic MR swine model (n=10). The progression of heart disease was followed for three weeks and TTE images were collected weekly. Figure 2A and Figure 2B show a representative TTE image prior to the terminal procedure (at three weeks of MR) with a flailing leaflet highlighted in pink and the corresponding regurgitant jet. The vena contracta width (Figure 2B, black line), or the narrowest segment of the regurgitant jet inside the LA, was used as a measure of regurgitation severity.
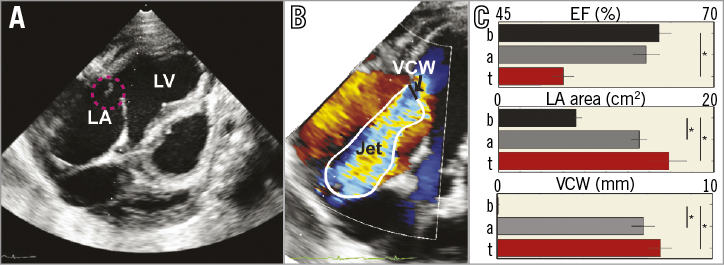
Figure 2. Chronic mitral regurgitation (MR) model in swine. A) Representative transthoracic echocardiographic image of a mitral valve closing irregularly (pink circle) after chordae tendineae disruption. B) Doppler flow recording of MR. C) Summary (n=10) of the ejection fraction (EF), left atrial (LA) area, and vena contracta width (VCW) at baseline (b), immediately after acute injury (a), and on terminal (t) transseptal access procedure day. * p<0.05.
Figure 2C summarises functional and structural changes from the MR model at baseline (B), after acute injury (A), and on the terminal procedure day (T; 22±2 days). MR caused a decrease in ejection fraction (EF; 63.6±1.4% baseline versus 52.5±1.2% terminal procedure, p=1.4×10–4), an increase in LA size (7.6±0.5 cm2 baseline versus 15.8±1.7 cm2 terminal procedure, p=1.2×10–4), and a large vena contracta width (7.6±0.5 mm terminal procedure). The European Association of Echocardiography grades MR as severe when the vena contracta width is greater than 7.0 mm25. On the terminal procedure day, the LV free wall and septum had not remodelled and their thicknesses were not statistically different compared to baseline values. These structural and functional changes were similar to the inclusion criteria for the Endovascular Valve Edge-to-Edge Repair Study (EVEREST II) that investigated catheter-based mitral valve repair with the MitraClip11.
IN VIVO SAFETY AND EFFICACY
Safety was assessed for the following: 1) procedure complications, 2) procedure time, 3) fluoroscopy time, 4) total fluoroscopic energy emitted, and 5) FO movement during puncture. Neither the TSAC (0 of 5) nor the CTD (0 of 5) had an inadvertent puncture that led to an adverse event such as an aortic root puncture or tamponade.
The procedure time, the fluoroscopy time, and the total fluoroscopic energy emitted were not statistically different between the TSAC and the CTD (Figure 3). The procedure time, fluoroscopy time, and the total fluoroscopic energy used for the TSAC versus the CTD were 5.22±1.35 min versus 5.08±1.27 min (p=0.89), 4.18±1.07 min versus 3.59±0.71 min (p=0.83), and 0.58±0.12 mGy·m2 versus 0.48±0.08 mGy·m2 (p=0.48), respectively. Utilisation of suction with the TSAC did not interrupt the clinical workflow for TS punctures and did not impact on key temporal and X-ray safety parameters.
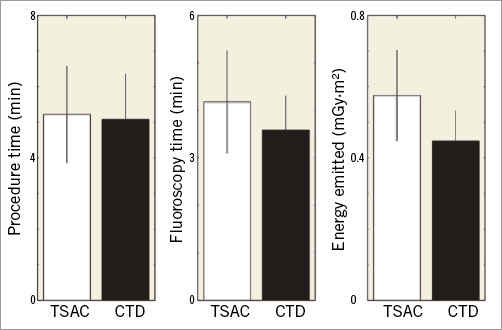
Figure 3. Procedure time and X-ray exposure when using the transseptal access catheter (TSAC) and conventional transseptal device (CTD) during a TSA procedure. There were no statistically significant differences. CTD: conventional transseptal device; TSAC: transseptal access catheter
Safety was also evaluated by quantifying tissue displacement during TS punctures. Significant interatrial septal displacement can cause the TS needle to perforate the LA unintentionally and thus minimising interatrial septal movement will probably reduce complications. Figure 4A-Figure 4C highlights the differences in interatrial septum deformation with the TSAC and the CTD. When suction was applied with the TSAC, the interatrial septum was stabilised, reducing tissue displacement (3.60±0.19 mm versus 8.7±0.30 mm, p=7.8×10–7) and curvature (0.7±0.13 cm–1 versus 4.2±0.37 cm–1, p=2.4×10–5) compared to the CTD. Although puncturing the FO with the CTD led to no adverse events (0 of 5), the device caused severe tissue deformation (Figure 4B), that brought the needle in close proximity to the posterior wall of the LA.
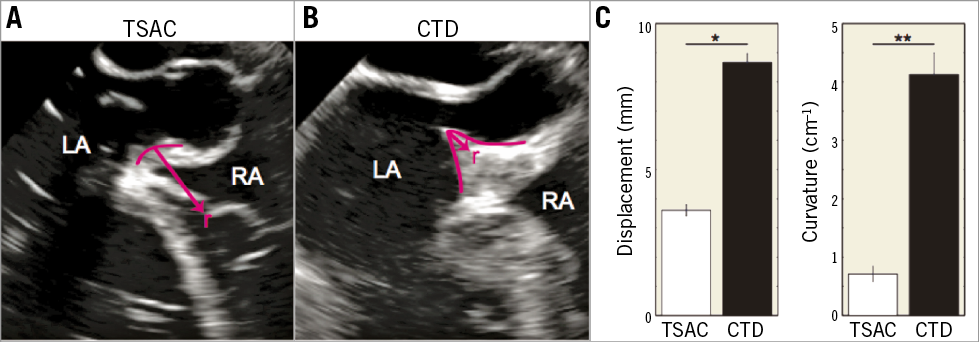
Figure 4. Interatrial septum (IAS) movement during transseptal puncture. A) With the transseptal access catheter (TSAC). B) With a conventional transseptal device (CTD). Pink lines highlight the IAS during puncture, where r is the radius of the circle that is fitted to the curved tissue. Curvature is 1/r. C) Summary of the tissue displacement (left) and curvature (right) during puncture. *p=2.4×10–5. **p=7.8×10–7. CTD: conventional transseptal device; IAS: interatrial septum; LA: left atrium; RA: right atrium; TSAC: transseptal access catheter
Efficacy was evaluated by examining the accuracy of the TS puncture in the chronic MR swine hearts, where the centroid of the FO was the target. A representative FO with a TS puncture is shown in Figure 5A and the accuracy quantification method is shown in Figure 5B. The average MR FO area was 2.2±0.27 cm2 (healthy swine, 1.5±0.27 cm2, p=0.08) and each MR FO differed in morphology. To negate these morphological differences between FO, the distance between the centroid and the puncture site (Figure 5B, D2 - solid arrow) was normalised to half the length of the major axis of the FO (Figure 5B, D1 - dashed arrow). The TSAC was found to be more accurate (0.57±0.10) as compared to the standard device (1.02±0.11, p=0.02).
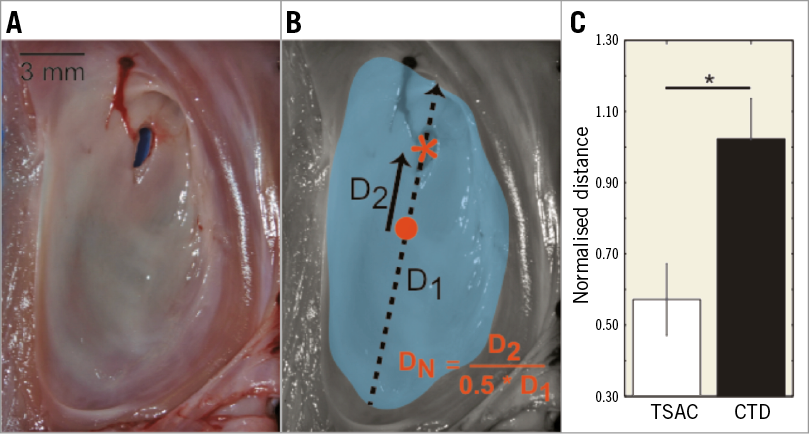
Figure 5. Fossa ovalis (FO) puncture accuracy. A) Representative FO and transseptal puncture. B) Quantification of puncture accuracy. FO - teal, FO centroid - orange circle, puncture site - orange asterisk, D1 - major axis of FO (dashed arrow), D2 - distance from puncture to centroid (solid arrow), DN – normalised distance. C) Normalised puncture distance with transseptal access catheter (TSAC) versus conventional transseptal device (CTD). *p=0.02. CTD: conventional transseptal device; FO: fossa ovalis; TSAC: transseptal access catheter
Discussion
This is the first TSA device that utilises suction to secure the FO during TS punctures. Our data show that: 1) the TSAC provides 6.5-fold greater stabilising suction force than needle puncture force; 2) the TSAC does not interrupt the procedure workflow of TSA and thus has similar procedure time and X-ray exposure compared to the CTD; 3) the TSAC reduces interatrial septum displacement and tenting; and 4) the TSAC facilitates a more accurate puncture. Overall, the TSAC provided a safe and reliable technique for TSA in normal and chronic MR swine.
For experienced electrophysiologists, TSA complications, such as aortic root perforations or tamponade, are uncommon, with reported rates of less than 1.0%15. An accurate TS puncture that targets regions within the FO is difficult, which is due to several confounding factors including the constant motion of the beating heart, the lubricious surface of the FO, and the variable thickness within the FO from structural remodelling. Even when guided to the correct position with TEE, the TS needle can drift or deflect to a position of lowest energy that differs from the original target. Here, we show in a chronic MR swine model that applying suction to the FO reduces needle drift and provides a 1.75-fold more accurate puncture. The CTD device that we tested had a normalised puncture distance of 1.0, which signifies that the needle punctured the edge of the FO. When forward pressure was applied, the needle would drift to the limbus of the FO, where it was constrained and finally perforated the tissue26. Although the CTD did not cause any complications, it was unable to make accurate punctures. The TSAC consistently punctured through the interior of the FO and was not limited to puncturing the edge of the FO.
Surgical repair of the mitral valve is predominately used to treat MR; however, over half of the patients with severe MR are not eligible for surgery because of age, comorbidities, and/or impaired LV function. In the USA alone, there are an estimated four million patients with severe MR (3+ or 4+)27, and thus a less invasive TS approach is required for a large number of these patients. Unfortunately, percutaneous mitral valve repair is hampered by long procedure times (can be >200 min), which is partially due to an initial inaccurate TS puncture that can result in the device being withdrawn from the LA and then reinserted across the FO8,10. The TSAC improved TS puncture accuracy in swine with severe MR (vena contracta width >7.0 mm)25. This improved accuracy may translate into more efficient procedures, especially those that rely on site-specific TS punctures including mitral valve repair, LAA closure, and paravalvular leak closure28.
Gross examination of FO from the TSAC-assisted group revealed 20% (one of five) had acute haemorrhage on the right atrial side of the FO. The TSAC utilises wall vacuum (300 mmHg) in short durations that total <5 min of suction time. In comparison, coronary artery bypass graft surgery with the Octopus® Tissue Stabilizer (Medtronic, Minneapolis, MN, USA) immobilises cardiac tissue with 400 mmHg and has a duration of 30-45 min29-32. Borst and colleagues29 pioneered off-pump coronary artery bypass surgery with the Octopus and showed that acute suction lesions were healed by six weeks. Similarly, TS puncture sites close and heal over time, and hence acute FO injury by the TSAC is probably temporary, but this needs to be assessed further.
Limitations
There are several limitations to this study. First, echocardiographic data were collected as two-dimensional images. Echocardiographic images were taken in the same imaging plane between experiments so that analysis of parameters was consistent. Second, we targeted the centre of the FO for TS access, whereas some interventional procedures require other positions (e.g., posterior, superior, inferior) for TS punctures. The centre of the FO was chosen because swine have small FO, something which prevented regional targeting of the FO. The FO of adult human hearts have been estimated to be 2.0 cm in diameter26,33, which will enable the 8 mm TSAC cup to be regionally positioned. Third, TSA in an MR swine model does not completely represent the puncturing conditions in diseased human tissue. To assess how the structural variability of FO can impact on TS punctures, we evaluated puncture force in the FO of human hearts. We determined that the puncture force was 1.17 N, which compares well to the 2.0 N reported by Howard et al33, and is well below the stabilising force produced by the TSAC (–7.73 N). There are always challenges to translation of animal preclinical experience into clinical practice and this merits future exploration. Future studies will need to evaluate the TSAC in patients with aneurysmal FO and those that resist mechanical punctures. Finally, given the improvements in imaging technologies (e.g., 3D echocardiography) to make the TSA procedure safer and more predictable, the utility of the proposed technology may be of more value to the inexperienced transseptal operators.
Conclusion
The suction transseptal access catheter stabilises and localises the FO during TS puncture to improve control and enhance puncture accuracy.
| Impact on daily practice Percutaneous structural heart therapies, including mitral valve repair and atrial appendage closure, require site-specific transseptal puncture of the fossa ovalis. Here, we describe a novel transseptal access catheter that utilises suction for precise control and improved transseptal puncture accuracy. In clinical practice, an accurate transseptal puncture would allow easier device placement and enhanced execution of left-sided procedures. |
Funding
This work was funded in part by National Institute of Health grant R43 HL123186 and 3DT Holdings.
Conflict of interest statement
M. Sulkin and Z. Berwick are employed by 3DT Holdings. G. Kassab is the founder and J. Navia is a co-inventor. The other author has no conflicts of interest to declare.

