Abstract
Background: An inferoposterior transseptal puncture (TSP) is generally recommended for percutaneous left atrial appendage (LAA) closure. However, the LAA is a highly variable anatomical structure. This may have an impact on the preferred TSP site.
Aims: This study aimed to determine the optimal TSP site for percutaneous LAA closure in different LAA morphologies.
Methods: In this prospective study, 182 patients undergoing percutaneous LAA closure were included. The spatial relationship of the LAA to the fossa ovalis and its consequence for TSP was assessed at preprocedural cardiac computed tomography (CCT).
Results: Based on CCT analysis, it was predicted that coaxial alignment between the delivery sheath and the LAA would be obtained by an inferoposterior, inferocentral, or inferoanterior TSP in 75%, 16% and 8% of cases, respectively. This was also confirmed by procedural LAA angiogram in 175 cases (96%) with <30° angle between the delivery sheath and the LAA central axis. Multivariate logistic regression analysis identified reverse chicken wing LAA (odds ratio [OR] 6.36 [1.85-29.3]; p=0.005) and posterior bending of the proximal LAA (OR 17.2 [3.3-96.2]; p<0.001) as independent predictors of a central or anterior TSP – this to increase the chance of obtaining coaxial alignment between the delivery sheath and the LAA.
Conclusions: An inferoposterior TSP is recommended in the majority of percutaneous LAA closure procedures in order to obtain coaxial alignment between the delivery sheath and the LAA. An inferior but more central/anterior TSP should be recommended in case of a reverse chicken wing LAA or posterior bending of the proximal LAA, which occurs in 20-25% of cases.
Introduction
Percutaneous transcatheter left atrial appendage (LAA) closure can be an alternative to chronic oral anticoagulation (OAC) as stroke prevention in patients with non-valvular atrial fibrillation (NVAF) and a high stroke and bleeding risk or contraindications to OAC therapy1234. In order to secure a safe and effective LAA closure procedure, careful sheath and device manipulation, as well as correct LAA closure device size selection, is of critical importance.
In order to implant a device into the LAA by the transfemoral transvenous approach, a transseptal puncture (TSP) has to be performed. A general consensus exists prescribing that an inferior and posterior puncture of the fossa ovalis should be aimed for in order to facilitate delivery of the LAA closure device into the LAA56. This also makes sense as the LAA is typically located superior and anterolateral in the left atrium (LA) and the LAA long axis is typically oriented anteriorly78.
However, the LAA is a highly variable anatomical structure in humans910. Consequently, a classic inferoposterior TSP does not always provide operators with the greatest ability to obtain co-axial alignment between the delivery sheath and the LAA central axis. Importantly, prior studies have also described that suboptimal device alignment (off-axis at the landing zone) carries a higher risk of incomplete LAA closure – with residual leakage into the LAA11.
To date, no studies have investigated the spatial relationship of the LAA to the fossa ovalis and its possible consequence for TSP. Hence, this is the first cardiac computed tomography (CCT)-based study aimed at determining the optimal TSP site for LAA closure in different types of LAA morphology and to identify independent predictors of a non-classic TSP site, if any.
Methods
Study population
In this prospective study, 182 consecutive patients with a preprocedural CCT and undergoing percutaneous LAA closure in the period 2019-2021 were included. All patients were known to have non-valvular atrial fibrillation (NVAF) and a high risk for stroke and bleeding and/or contraindication(s) to OAC therapy. All procedures were performed under local anaesthesia with intracardiac echocardiography guidance and using one of the following CE-mark approved LAA closure devices: AMPLATZER Amulet (Abbott Laboratories) and Watchman FLX (Boston Scientific). All patients gave written informed consent for the procedure and the use of anonymous data for clinical research. All baseline patient and procedural data were collected prospectively in the Copenhagen LAA Registry.
Cardiac computed tomography (CCT) acquisition and analysis
All patients underwent a multidetector CCT scan before the procedure. CCT data acquisition was performed in accordance with a site-specific protocol used for CCT imaging in preparation for percutaneous LAA closure, as published earlier12. CCT data acquisition was electrocardiography (ECG)-gated, contrast-enhanced and performed with 0.5 mm slice thickness and 0.5 mm increments.
First, quantitative assessments of left atrial (LA), right atrial (RA), left ventricular (LV) and right ventricular (RV) diastolic volumes were made on an external workstation (Vitrea 6.3; Vital Images Inc.) in the mid-diastolic phase of the heart cycle, as previously described13. LA and RA volumes (including appendage, but carefully excluding pulmonary veins and vena cava) were assessed manually by tracing the endocardial borders on 15-20 tomographic slices. LV and RV diastolic volumes were derived as manually corrected automated delineation of the endocardial borders.
Second, the LAA morphology was assessed for each patient and classified into one of three types: non-angulated (windsock, cactus, and cauliflower), chicken wing, and reverse chicken wing LAA. The chicken wing morphology was defined as an obvious bend in the proximal or middle part of the dominant LAA lobe; in cases where the dominant LAA lobe bent posteriorly, the LAA was classified as reverse chicken wing. Next, the dimensions of the LAA ostium and LAA landing zone – at a depth of 10 mm distal to the ostium – were measured. LAA-specific CCT measurements were made using the LAA workflow of 3mensio software (Pie Medical Imaging).
Third, the anatomical relationship between the fossa ovalis and LAA was assessed. The fossa ovalis was defined using the LAA Septal Crossing workflow of 3mensio software. As shown in Figure 1, the lateral view (RAO 45°) was used to assess the co-axial alignment with the LAA central axis following an anterior versus posterior TSP – by measuring the angle (<180°) formed by an anterior or posterior puncture of the fossa ovalis and the proximal LAA central axis, the latter connecting the centres of the LAA ostium and LAA landing zone. The apical view (LAO 45°) was used to assess the alignment with the proximal LAA central axis following a superior versus inferior TSP – by measuring the angle (<180°) formed by a superior or inferior puncture of the fossa ovalis and the LAA central axis. The closer this angle is to 180°, the more this TSP site is in line with the proximal LAA central axis and, hence, should be considered the preferred TSP site for this patient’s LAA anatomy.
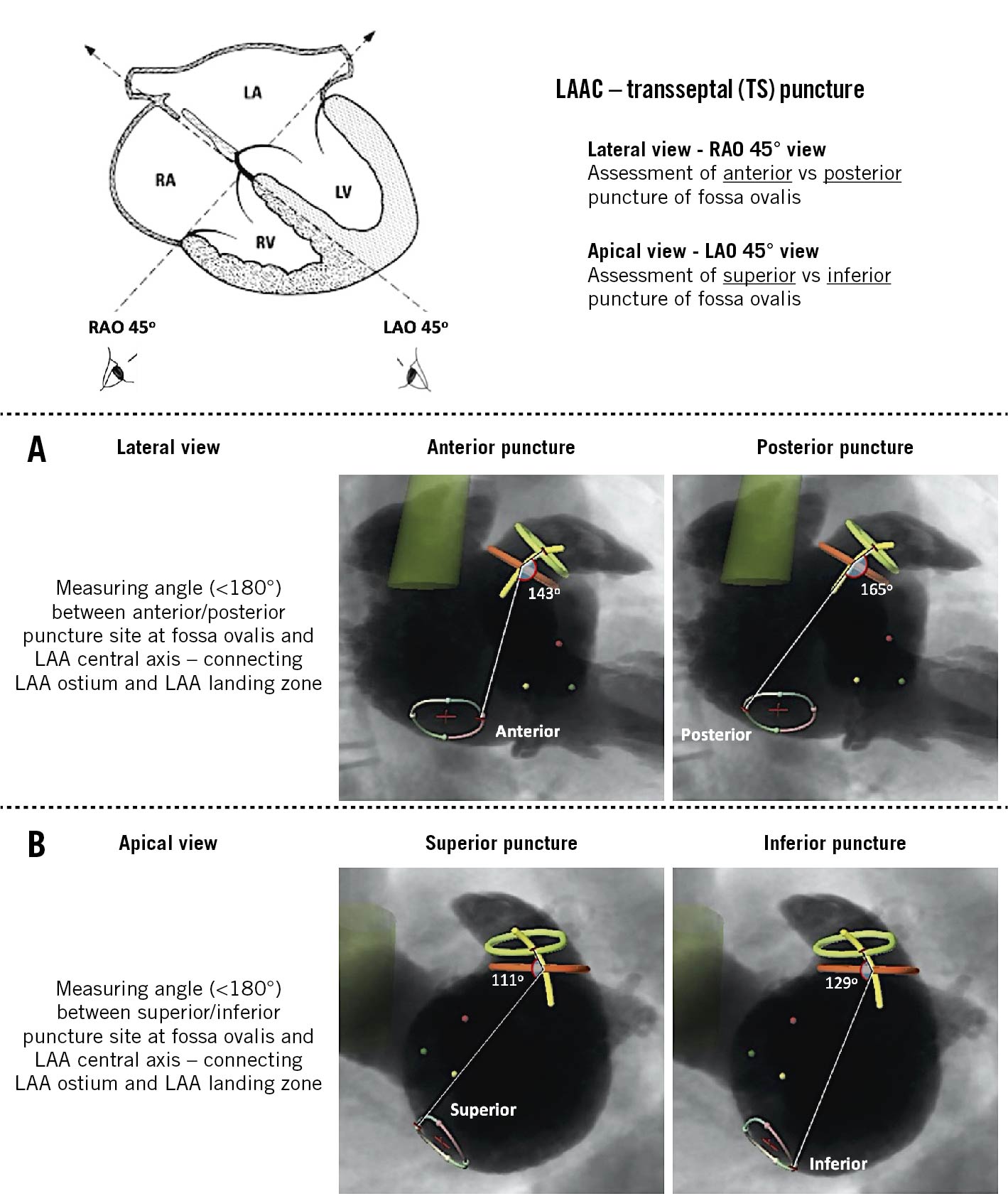
Figure 1. Anatomical relationship between the fossa ovalis and the LAA. A) The lateral view (RAO 45°) was used to assess co-axial alignment with the LAA central axis following an anterior versus posterior TSP, by measuring the angle (<180°) formed by an anterior or posterior puncture at the fossa ovalis and the proximal LAA central axis. B) The apical view (LAO 45°) was used to assess co-axial alignment with the LAA central axis following a superior versus inferior TSP, by measuring the angle (<180°) formed by a superior or inferior puncture at the fossa ovalis and the LAA central axis. The closer this angle is to 180°, the more this TSP site is in line with the proximal LAA central axis and, hence, should be considered the preferred TSP site for this particular anatomy. LAAC: left atrial appendage closure; LAO: left anterior oblique; RAO: right anterior oblique; TSP: transseptal puncture
Fourth, orientation of the proximal LAA was assessed in the lateral view. The angle between the LAA ostium and proximal LAA central axis was systematically measured at the side of the left circumflex artery, the proximal LAA central axis was again defined as the line connecting the centres of the LAA ostium and LAA landing zone. This latter CCT analysis was performed by using the LAA workflow of 3mensio software.
Procedural LAA angiogram
In this series of LAA closure procedures, the TSP site was chosen and TSP was performed as predefined at preprocedural CCT analysis. The angle between the delivery sheath (distal end) and the proximal LAA central axis was assessed and measured at the procedural LAA angiogram, before deployment of the LAA closure device. In the case of <30° angle, the TSP was considered successful with co-axial alignment between the delivery sheath and LAA central axis.
Statistical analysis
Categorical variables are reported as absolute values and percentages. Continuous variables are reported as means±standard deviation. The mean optimal predicted TSP site at the posterior-anterior axis for three different LAA morphologies (non-angulated, chicken wing, reverse chicken wing) was compared using a one-way analysis of variance (ANOVA). In order to identify possible independent predictors of a non-classic, non-inferoposterior TSP, all variables with p≤0.10 on univariate analysis were included in a stepwise multivariate logistic regression model. The results of these analyses are reported as odds ratios (OR) with 95% confidence intervals (CI). Statistical significance was defined as a p-value <0.05. All statistical analyses were performed with SPSS software, Version 24 (IBM Corp.).
Results
Patient population
A total of 182 consecutive patients who had undergone preprocedural CCT and percutaneous LAA closure in the period 2019-2021 were included in this study. Demographic and baseline data are summarised in Table 1. The distribution of non-angulated, chicken wing and reverse chicken wing LAA morphology was 48%, 39% and 13%, respectively.
Prediction of optimal TSP site
Based on the described CCT analysis, the optimal TSP site could be predicted and calculated for every individual patient (Figure 2A). A scatter plot showing the distribution of the optimal TSP site for all patients included in this study (N=182) is shown in Figure 2B.
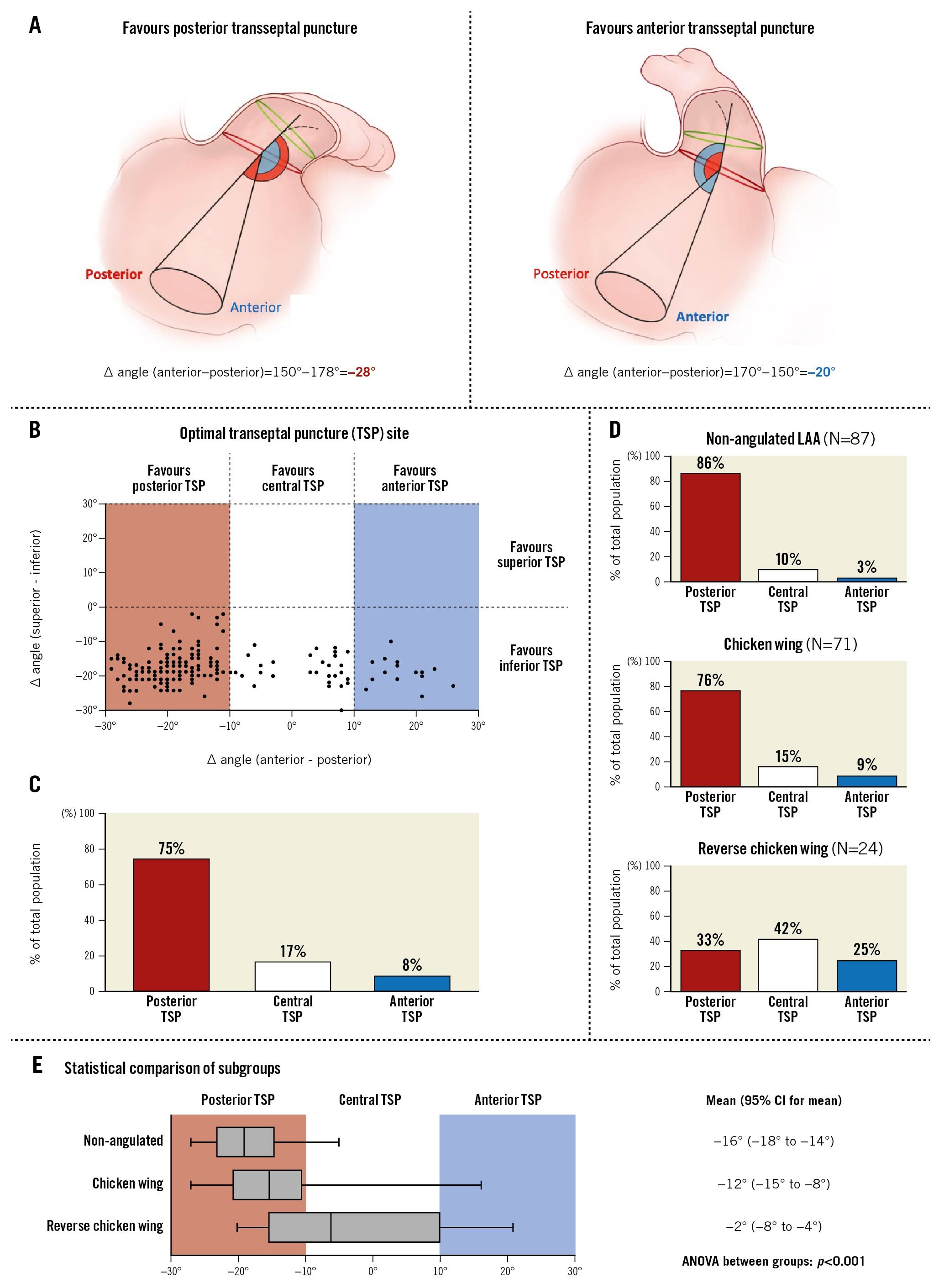
Figure 2. CCT-based prediction of optimal TSP site. A) Schematic images illustrating the calculation of ∆ angle (anterior - posterior) in the lateral view. B) Scatter plot showing the distribution of the optimal TSP site for all patients along the anterior-posterior axis (X-axis) and superior-inferior axis (Y-axis). C) Distribution of a preferred posterior, central and inferior TSP in the entire study population. D) Distribution of a preferred posterior, central and inferior TSP in the three morphological subgroups. E) Preferred TSP site comparison between the three LAA morphological subgroups, showing box-and-whisker plots (box, 25th and 75th percentile; whiskers, 5th and 95th percentiles) and one-way ANOVA comparing the means. CCT: cardiac computed tomography; LAA: left atrial appendage; TSP: transseptal puncture
For all patients, co-axial alignment between the delivery sheath and the LAA central axis was predicted to be obtained by an inferior TSP (100%). Along the posterior-anterior axis, there was a wider spread of optimal TSP sites (Supplementary Figure 1). Consequently, the fossa ovalis was subdivided into a posterior, central and anterior zone corresponding to an ∆ angle (anterior–posterior) of (−30° to −10°), (−10° to 10°), and (10° to 30°), respectively (Figure 2A). Co-axial alignment between the delivery sheath and proximal LAA central axis was predicted to be obtained by a posterior, central, or anterior TSP in 75%, 17%, and 8% of patients, respectively (Figure 2C). This was also confirmed by procedural LAA angiogram in 175 cases (96%) with <30° angle between the LAA closure device delivery sheath and proximal LAA central axis.
The mean optimal TSP site along the posterior-anterior axis was significantly different between the three different LAA morphologies (Figure 2D, Figure 2E) (ANOVA between groups: p<0.001). Transseptal crossing at the central or anterior part of the fossa ovalis should be considered in two-thirds of cases with a reverse chicken wing LAA morphology.
Association between proximal LAA orientation and optimal TSP site
The orientation of the proximal LAA was assessed in the lateral view, indicating whether the proximal LAA had a predominantly anterior (<90°) or posterior (≥90°) orientation (Figure 3).
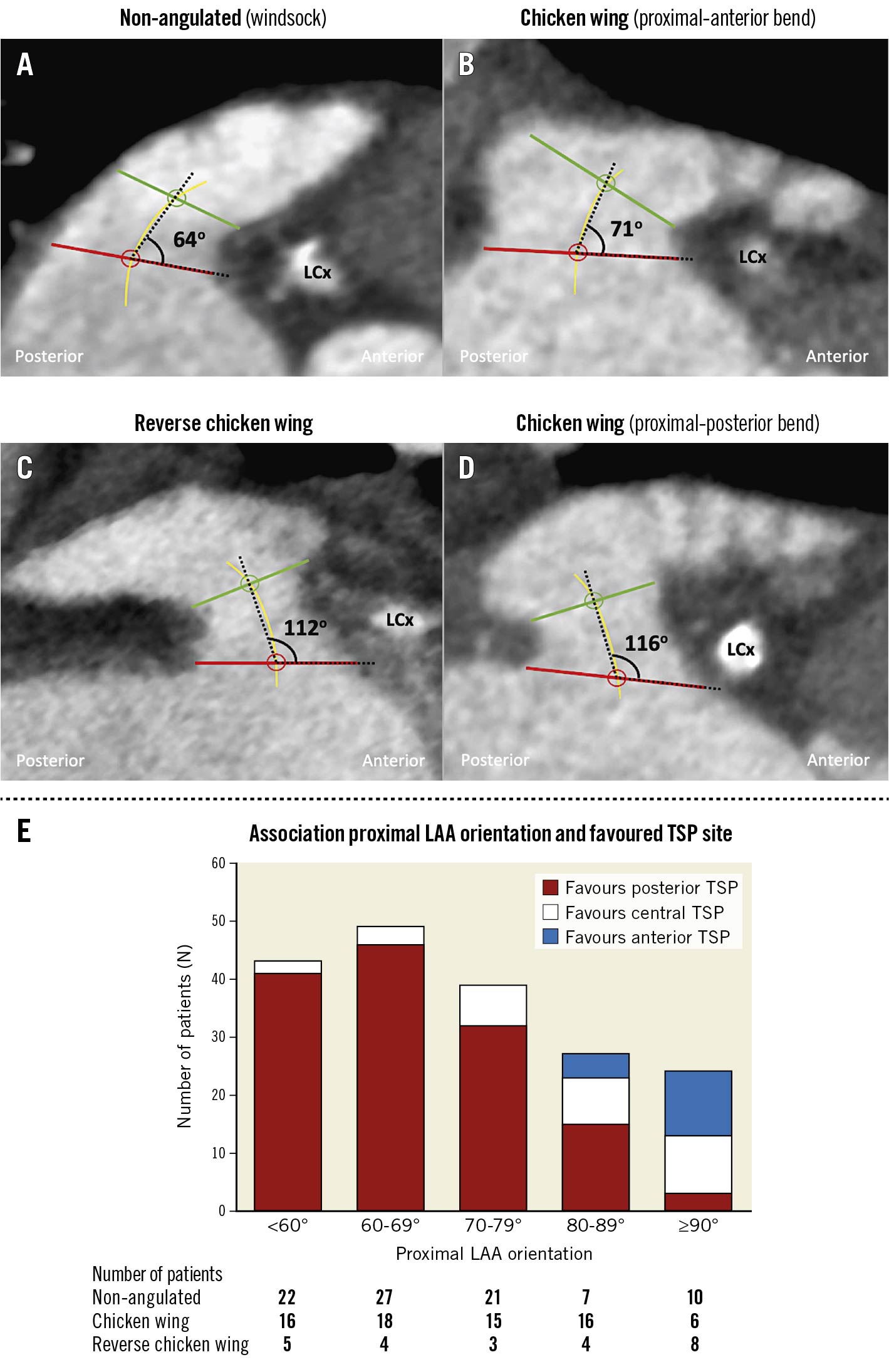
Figure 3. Association between proximal LAA orientation and optimal TSP site. A) - D) The orientation of the proximal LAA was assessed in the lateral view, indicating whether the proximal LAA had a predominantly anterior orientation (A & B) or posterior orientation (C & D). The angle between the LAA ostium and proximal LAA central axis was systematically measured at the side of the left circumflex artery; the proximal LAA central axis was defined as the line connecting the centres of the LAA ostium and LAA landing zone. E) A posterior TSP is the best option in case of a more anterior orientation of the proximal LAA (<80°), whereas a non-classic, central-anterior TSP should be considered in LAAs with an LAA ostium-LAA central axis angle ≥80°. A posterior orientation of the proximal LAA was observed relatively more often in reverse chicken wing LAAs. LAA: left atrial appendage; LCx: left circumflex TSP: transseptal puncture
The majority of patients had an anterior orientation of the proximal LAA (N=158, 87%); only 24 patients (13%) had a posteriorly oriented proximal LAA. A posterior orientation of the proximal LAA was observed relatively more often in reverse chicken wing LAAs (8/24, 33%). Also, LAAs with a classic chicken wing morphology were sometimes found to have a posterior bend in their proximal LAA , favouring a more central anterior transseptal crossing. A posterior TSP should be preferred in case of a predominantly anterior orientation of the proximal LAA (<80°), whereas a central-anterior TSP could be the better option in LAAs with an LAA ostium-LAA central axis angle ≥80° (N=33/51, 65%) (Figure 3).
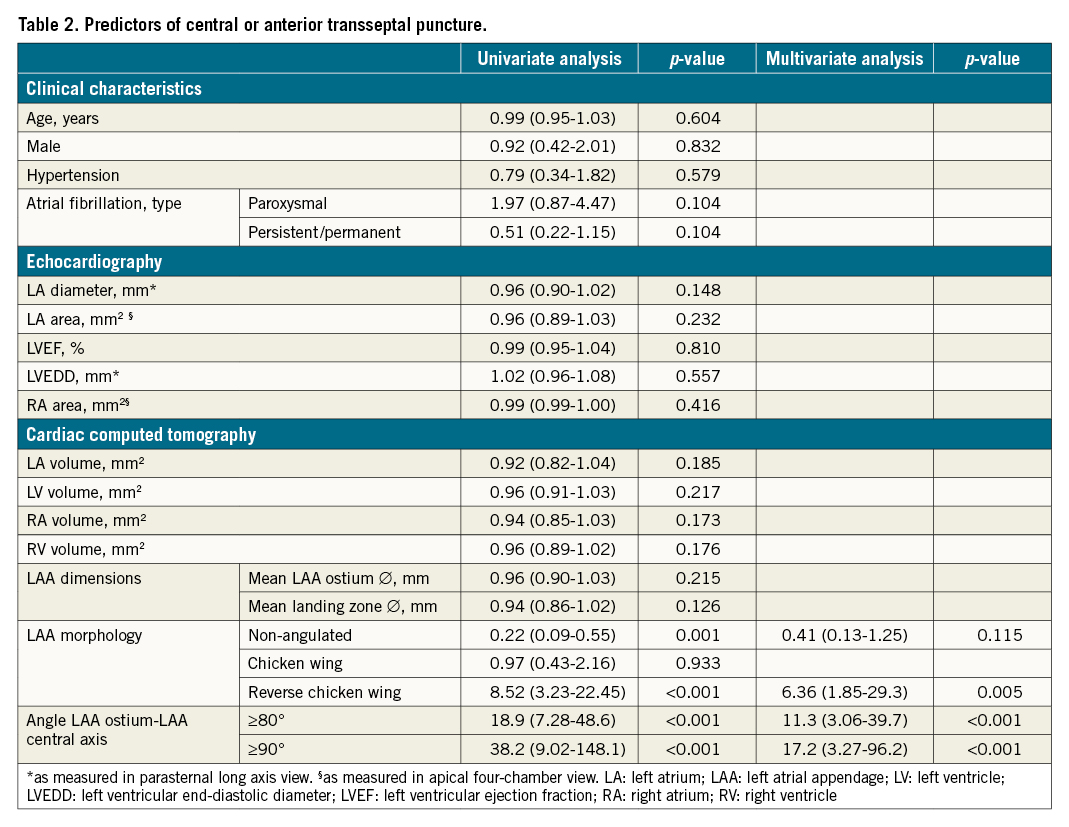
Predictors of a central-anterior TSP site
Based on a stepwise multivariate logistic regression analysis, including a wide range of clinical, echocardiographic and CCT characteristics (Table 2), the only independent variables predicting a proximal LAA central axis alignment with a central-anterior TSP were a reverse chicken wing LAA morphology (OR 6.36; p=0.005) and an LAA ostium-LAA central axis angle ≥80° (OR 11.3; p<0.001) and ≥90° (OR 17.2; p<0.001).
Discussion
This is the first study investigating the spatial relationship of the LAA to the fossa ovalis and its possible consequence for TSP during percutaneous LAA closure. In brief, our results indicate that the fossa ovalis is best punctured inferoposterior in 75-80% of cases, in order to have the best chance of obtaining co-axial alignment between the delivery sheath and the LAA central axis. An inferior but more central-anterior TSP should be considered in 20-25% of procedures, especially in cases with a reverse chicken wing LAA or posterior bending of the proximal LAA. A superior TSP should be avoided in all cases (Central illustration).
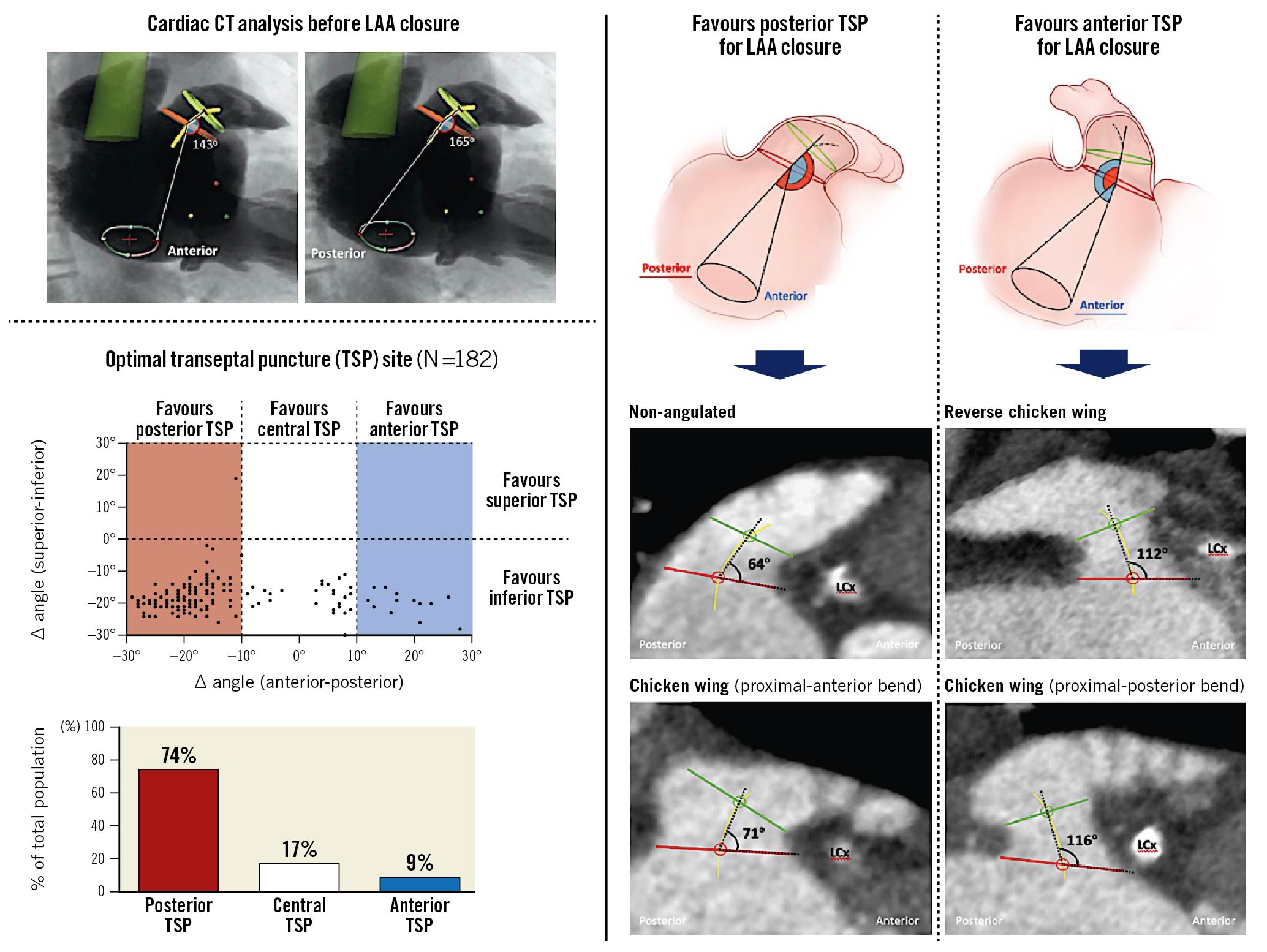
Central illustration. Cardiac computed tomography-based selection of transseptal puncture (TSP) site for percutaneous left atrial appendage (LAA) closure. An inferoposterior TSP is recommended in the majority of percutaneous LAA closure procedures in order to obtain co-axial alignment between delivery sheath and LAA; an inferior but more central/anterior TSP should be recommended in the case of a reverse chicken wing LAA or posterior bending of the proximal LAA, which occurs in 20-25% of cases.
Typically, the LAA is located in the superior and anterolateral aspect of the LA and the long axis of the LAA is oriented anteriorly78. Consequently, it has been recommended (and has been common practice) to aim for an inferoposterior TSP when performing LAA closure. In comparison, a more superior-posterior TSP is targeted when performing a MitraClip (Abbott) procedure, whereas a more anterior TSP is recommended when engaging in a pulmonary vein isolation56.
The LAA is a highly variable anatomical structure between patients which does not only vary in its dimensions but also in the number of lobes as well as in the shape and orientation of these lobe(s)910. Clearly, this may have an impact on the TSP site to be targeted, as operators strive for co-axial alignment between the delivery sheath and proximal LAA central axis. In case of suboptimal co-axial alignment, an LAA closure procedure may become very challenging and the risk of off-axis device implantation, with risk of peri-device leakage11, as well as procedural complications may increase markedly.
Traditionally, imaging and sizing of the LAA has relied on transoesophageal echocardiography (TEE)1415. However, in parallel with the acceptance of CCT as the “gold standard” imaging tool to prepare for transcatheter aortic valve implantation, CCT is also increasingly recognised as a valuable imaging modality to prepare for percutaneous LAA closure12161718. The possibility of easily generating three-dimensional multiplane reconstructions not only results in more accurate and reproducible measurements of the LAA dimensions but also offers the opportunity to study in detail the spatial relationship between the LAA and fossa ovalis and its possible consequence for TSP.
As the WATCHMAN FLX and AMPLATZER Amulet LAA closure devices are designed to be implanted in the proximal part of the LAA (at the landing zone), co-axial alignment with the proximal LAA central axis should be aimed for19. In accordance with common practice, this study found that an inferoposterior TSP results in co-axial alignment between the delivery sheath and the proximal LAA in the majority of patients (75-80%). Independent predictors of an inferior but more central-anterior TSP were found to be a reverse chicken wing LAA and an angle between the LAA ostium and proximal LAA central axis ≥80/90°, which was found in 20-25% of patients. Therefore, it is important to make a distinction between the “overall” orientation of the LAA and the orientation of the proximal LAA. Figure 3D illustrates an “overall” anteriorly oriented chicken wing LAA in which the proximal part bends posteriorly, favouring a more central-anterior TSP. Appreciating this aspect preprocedurally will not only result in a less complex procedure, but will also increase the chance of implanting the LAA closure device in line with the LAA central axis and, hence, obtain complete LAA closure without any peri-device leak. This should also become the new criterion in order to label an LAA closure procedure “successful” in the future.
Although many centres have adopted CCT as a routine investigation in their preprocedural planning for LAA closure19, it is not yet a routine assessment to delineate the fossa ovalis. The latter assessment is also not always easy, as it requires high-quality CCT with also a minimum of contrast at the right atrial side. Therefore, detecting a reverse chicken wing LAA and/or measuring an angle ≥80° between the LAA ostium and LAA proximal central axis should flag the possibility that a non-classic, more central/anterior TSP may be the better option to obtain co-axial alignment between the delivery sheath and LAA central axis. An example of how comprehensive preprocedural planning, including optimal TSP site assessment, can reduce procedural complexity and improve its outcome can be found in Supplementary Figure 2.
Despite the usefulness of CCT in preprocedural planning, it is important to emphasise that echocardiographic imaging during the LAA closure procedure is still recommended – either by intracardiac echocardiography or by (mini- or micro-multiplane) TEE. This is merely to assess the interatrial septum (whether lipomatous, aneurysmal or patent foramen ovale is present) and guide the TSP and LAA closure device implantation.
In conclusion, although this study presents strong data supporting the use of CCT in the preprocedural planning for LAA closure, a randomised controlled trial comparing a standard (TEE) and CCT-based preprocedural planning of percutaneous LAA closure should be encouraged. Whether such a trial will ever be conducted is doubtful. However, dedicated structural heart interventionalists with skills in CCT analysis are becoming the new standard. The use of CCT in preprocedural planning (replacing and/or supplementing TEE, depending on which kind of structural heart intervention is planned) is more often becoming routine practice. This now has been even further accelerated by the current COVID-19 pandemic.
Limitations
The inability to incorporate a pre-shaped delivery sheath into our CT analysis and assessment of the preferred TSP site was one of the main study limitations. The most recent 3mensio Structural Heart LAA software, version 10.0, includes a “Catheter Path Simulation” in the septal crossing module. Unfortunately, this feature did not generate reliable simulations in our experience. In addition, the analysis and findings reported in this study may theoretically be different for other LAA closure devices and/or delivery sheaths. Also, the arrival of steerable delivery sheaths may have an impact on the ease of obtaining co-axial alignment with the LAA central axis and eventually make the exact site of interatrial septum crossing less important. However, these data are still missing and will have to be collected in future registries and studies. Despite the above-mentioned limitations, this study investigated a topic which has never been studied before.
Conclusions
An inferoposterior TSP should be recommended in the majority of percutaneous LAA closure procedures in order to obtain co-axial alignment between the delivery sheath and proximal LAA. An inferior but more central-anterior TSP should be considered in the case of a reverse chicken wing LAA or a posterior bend of the proximal LAA, which occurs in 20-25% of cases. There is no doubt that an optimised preprocedural planning for percutaneous LAA closure will be one of the keys to a further optimisation and streamlining of this structural heart procedure. Larger multicentre studies will be needed to confirm these findings.
Impact on daily practice
An inferior and posterior transseptal puncture (TSP) has been generally recommended when performing percutaneous left atrial appendage (LAA) closure. An inferior but more central/anterior TSP should be recommended in the case of a reverse chicken wing LAA or a posterior bend of the proximal LAA, which occurs in 20-25% of cases. Preprocedural cardiac computed tomography analysis can help to determine the optimal TSP site.
Guest Editor
This paper was guest edited by Franz-Josef Neumann, MD; Department of Cardiology and Angiology II, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany.
Funding
M. Fukutomi is supported by a research grant from Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad.
Conflict of interest statement
G. Bieiliauskas has received consultant fees from Abbott, Boston Scientific and Medtronic. K. Kofoed has received institutional grants from AP Møller og hustru Chastine McKinney Møllers Fond, The Meyer Foundation, and Canon Medical Corporation. O. De Backer has received institutional research grants and consultant fees from Abbott and Boston Scientific. L. Søndergaard has received institutional research grants and consultant fees from Abbott, Boston Scientific and Medtronic. The Guest Editor reports lectures fees paid to his institution from Amgen, Bayer Healthcare, Biotronic, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis; consultancy fees paid to his institution from Boehringer Ingelheim; and grant support from Bayer Healthcare, Boston Scientific, Biotronic, Edwards Lifesciences, GlaxoSmithKline, Medtronic, and Pfizer. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

