
Abstract
Aims: Device surveillance after left atrial appendage (LAA) closure (LAAC) is important to assess device positioning, peri-device leak (PDL) and device-related thrombus (DRT). There are limited data on the role of cardiac CT angiography (CCTA) after LAAC. We therefore sought to compare CCTA to transoesophageal echocardiography (TEE) in patients who successfully underwent LAAC.
Methods and results: We report our consecutive series of non-valvular atrial fibrillation patients who underwent LAAC and had CCTA and TEE post LAAC. Prospective cardiac-gated CCTA was performed with the Toshiba 320-detector or Siemens second-generation 128-slice dual-source scanner, and post-processing was performed with IMPAX 3D reformats. Glomerular filtration rate <30 mL/min/1.73 m² was an exclusion for CCTA. Device positioning, PDL or fabric leak, ratio of left atrial (LA) to LAA linear attenuation coefficient, and DRT were analysed. One hundred and two patients underwent LAAC (79 WATCHMAN, 17 Amulet, 6 ACP). Mean age was 76.4±7.5 years, CHADS2 score 3.0±1.3, and CHADS-VASc score 4.6±1.6. CCTA was performed at a mean of 105.2±54.8 days, and TEE at a mean of 124.9±100.3 days post LAAC. LAA patency was observed in 52/100 (52%), with 45 (86.5%) via PDL and seven (13.5%) through fabric leak. Linear attenuation coefficient <100 HU and LA:LAA ratio <0.25 were seen in occluded devices. PDL was only observed in 35/102 (34.3%) on TEE. Mean device compression was greater with sealed devices (11.3±4.3% versus 8.2±4.0%, p<0.001). There was only one DRT, which was observed on both TEE and CCTA.
Conclusions: CCTA is a suitable alternative to TEE for device surveillance post LAAC. CCTA was more sensitive than TEE for assessing PDL and can delineate the cause of residual LAA contrast patency.
Introduction
The left atrial appendage (LAA) is the main source of thrombus in patients with non-valvular atrial fibrillation (AF) at high stroke risk1. Oral anticoagulation (OAC) is recommended to prevent thromboembolism. However, a significant proportion of patients have contraindications to OAC. LAA closure (LAAC) with the WATCHMAN™ device (Boston Scientific, Marlborough, MA, USA) has been shown to be safe and effective in preventing strokes2. The European Society of Cardiology guidelines assigned a class IIB recommendation for LAAC in patients with non-valvular AF at high stroke risk and with contraindication(s) to OAC3. It is anticipated that LAAC will increasingly play a major role in stroke prevention since AF prevalence is projected to more than double by 2050, especially in patients intolerant to OAC4.
To ensure the long-term efficacy of LAAC, routine follow-up device surveillance is important to assess device competency and complications. Adequate device compression and configuration is important for device stability and appendage sealing. Accurate, reproducible and reader-independent imaging with measurements of peri-device leak (PDL), device compression, and other characteristics can help to assess implant outcomes. Furthermore, identification of device-related thrombus (DRT) allows institution of anticoagulation to prevent thromboembolic complications. Conventionally, it is recommended that transoesophageal echocardiography (TEE) be performed post LAAC; however, TEE is invasive and the findings can be operator-dependent.
Given the superior spatial resolution and three-dimensional (3D) assessment with cardiac computed tomography angiography (CCTA), CCTA is increasingly used for baseline imaging and post-surveillance for LAAC5. Higher spatial resolution may allow better qualitative assessment of the mechanisms of incomplete LAA sealing. However, there are limited data comparing CCTA to TEE for diagnostic accuracy and the utility for post-LAAC surveillance. Therefore, we sought to compare CCTA to TEE in patients who successfully underwent LAAC.
Methods
We retrospectively analysed our consecutive series of patients who underwent endovascular LAAC at Vancouver General Hospital who had both TEE and CCTA performed post LAAC for surveillance. Indications for LAAC were non-valvular AF with high stroke risk (CHADS2 ≥1 and/or CHADS-VASc ≥2) and contraindications to OAC6,7. LAAC was performed as previously described8 with the commercially available AMPLATZER™ Cardiac Plug (ACP) or Amulet™ (second-generation ACP) (St. Jude Medical [now Abbott Vascular], St. Paul, MN, USA), or the WATCHMAN device. Baseline TEE and CCTA were performed to rule out LAA thrombus and to ensure suitable anatomy for LAAC. The study protocol was approved by the University of British Columbia Research Ethics Board.
In brief, LAAC procedures were performed under general anaesthesia utilising TEE guidance. Transseptal punctures were performed inferoposteriorly at the fossa ovalis. Saline loading to achieve mean left atrial pressure >12 mmHg9 and intravenous heparin were given to maintain an activated clotting time (ACT) >250 s. Device sizing was based upon measurements obtained from baseline CCTA and procedural TEE after volume loading. For device sizing, 2-6 mm upsizing was used above the largest diameter. For WATCHMAN, the PASS (Position, Anchor – tug test, Size – 8-20% compression, and Seal – <5 mm PDL) criteria were achieved prior to device release. For the ACP/Amulet, the five signs of CLOSE (Circumflex – lobe distal by 2/3, Lobe tyre-shaped compression, Orientation, Separation of lobe-disc, and Elliptical – concave disc) criteria were achieved prior to device release. A transthoracic echocardiogram was performed the next day, prior to discharge.
Post procedure, patients were treated primarily with aspirin and clopidogrel for one to three months, followed by aspirin indefinitely. Clinical follow-up was performed at three months and annually thereafter. Routine follow-up imaging was performed with TEE and/or CCTA at one to six months post LAAC (typically at three months). Glomerular filtration rate (GFR) <30 mL/min/1.73 m2 was an exclusion for CCTA. For this study, we included only patients who had both TEE and CCTA post LAAC for comparison of these imaging modalities.
Prospective systolic-triggered ECG-synchronised cardiac-gated CCTA was performed with the Aquilion ONE™ 320-detector (Toshiba Medical Systems Corporation, Tokyo, Japan) or the second-generation 128-slice dual-source CT, SOMATOM Definition Flash (Siemens Healthcare, Forchheim, Germany) scanners, and the digital images were interpreted with the Vitrea Workstation™ (Vital, a Toshiba Medical Systems Group Company, Zoetermeer, the Netherlands). Supplementary Table 1 describes the LAAC CT protocol at our institution. LAA imaging is obtained at greatest dimension with cardiac phase reconstruction at 30–40% of the R–R interval or ~250 ms after the R wave. Digital post-processing and reconstruction were performed with IMPAX 3D reformats to assess LAAC device positioning and the site of PDL. The PASS and CLOSE criteria were appraised. We used 3D multiplanar rendering with three planes locked at a 90-degree angle to reconstruct images. For ACP/Amulet, the axis markers were maintained perpendicular to the disc, keeping the centre of the axis aligned with the screw-hub centre. For the WATCHMAN, the maximum intensity projection images were used which allowed adequate visualisation of the entire nitinol frame of the device. The axis markers were kept perpendicular to the coves of the WATCHMAN parachute, while the centre of the axis was brought in line with the screw-hub centre. Both CCTA studies (S.R. Qamar and S. Nicolaou) and TEE images (M. Tsang and J. Saw) were interpreted by two readers with final consensus.
Device compression was calculated as: (manufacturer device diameter – largest measured diameter)/manufacturer device diameter x 100%. Both maximum and minimum device compressions (of device lobe) were measured, and these were averaged to produce the mean device compression. LAA patency was defined as residual contrast leak from the left atrium (LA) into the LAA, either along the side of the devices (PDL from ostial gap) or leak through the fabric of the device (“fabric leak” from diffusion of contrast through the non-endothelialised polyethylene terephthalate membrane). Residual contrast opacification of LAA was defined as increased attenuation of the LAA, with percentage of LAA opacification measured and categorised into <50% or >50% compared to the LA. Quantitative contrast assessment of LAA was carried out by measuring the average linear attenuation coefficient (Hounsfield units [HU]) in the LAA distal to the implanted device, using a circle diameter of 3 mm for the region of interest. The LAA was subsequently classified as patent or sealed using a cut-off of 100 HU (<100 HU was defined as sealed), as we had previously defined (Figure 1),5. The average contrast attenuation was further compared with the average contrast density in the LA. LA:LAA attenuation ratio was then calculated, with <0.25 categorised as thrombosed LAA and >0.35 as patent LAA. Ratios between 0.25 and 0.35 were considered equivocal; these CCTAs were further evaluated for direct evidence of contrast leak. The size of the PDL was classified as mild (<3 mm), moderate (3-5 mm), or large (≥5 mm) on CCTA or TEE. Additional findings of atrial-side DRT and pericardial effusion were assessed.
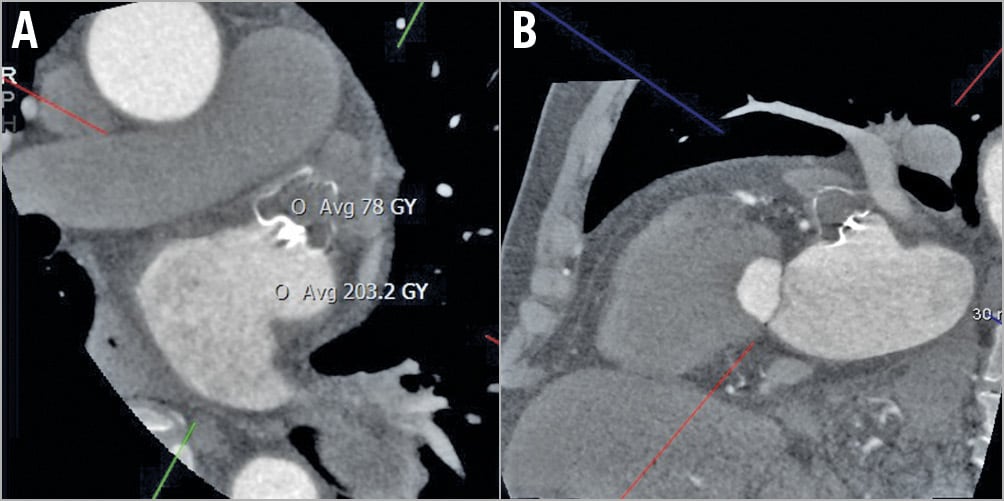
Figure 1. CCTA oblique multiplanar reformat images showing well-positioned WATCHMAN with complete occlusion of the LAA (<100 HU) (A & B).
STATISTICAL ANALYSIS
Baseline characteristics were detailed by descriptive statistical analysis. Continuous variables are presented as mean and standard deviation or median and interquartile range (IQR). Categorical data were summarised as frequency and percentage and were compared using Fisher’s exact test or the chi-square test. Average linear attenuation coefficients (HU) were analysed as dichotomous or continuous values with cut-offs to standardise the results. The Mann-Whitney U test was applied for comparison. Sensitivity and specificity was calculated. Pearson’s correlation coefficient was used to evaluate the association between PDL size measured on CCTA versus TEE. Data were analysed with Stata, version 14.2 (StataCorp, College Station, TX, USA).
Results
We report on 102 consecutive patients who underwent successful LAAC – 79 WATCHMAN, 17 Amulet, and 6 ACP. Baseline characteristics are described in Table 1. All patients had contraindications to long-term OAC. No major procedural or periprocedural complications occurred. During the procedure after device release, mild leak (<3 mm) was observed in 12 cases and moderate leak (3-5 mm) in two cases. No leak >5 mm was observed. Of these 14 procedural leaks, 6/14 had a persistent leak on follow-up TEE and 7/14 had LAA residual contrast opacification on CCTA. Post LAAC, the majority (94.1%) were discharged on DAPT. Follow-up CCTA was performed at 105.2±54.8 days (median 93 days, IQR 0-476 days), and TEE at 124.9±100.3 days (median 96 days, IQR 0-823 days) post LAAC. Mean radiation dose for CCTA was 5.1±3.9 mSv.
On follow-up imaging, the presence of LAA patency was observed in 52/102 (51.0%) on CCTA. Of these, 45 (86.5%) had PDL, and seven (13.5%) had fabric leak. The overall incidence of patency with the WATCHMAN was 41/79 (51.9%) and with the ACP/Amulet it was 11/23 (47.8%) (p=0.81). Fabric leak was only observed with WATCHMAN devices, occurring in 7/79 (8.9%) (Figure 2); the remainder of LAA patency was due to PDL (34/79, 43.0%). PDL size for the WATCHMAN was <3 mm in 27/34 (79.4%), 3-5 mm leak in 6/34 (17.6%), and ≥5 mm in 1/34 (2.9%) patients. PDL size with the ACP/Amulet was <3 mm in 4/11 (36.4%), 3-5 mm in 6/11 (54.5%), and ≥5 mm in 1/11 (9.1%). PDL was only present on TEE in 35/102 (34.3%) (p=0.016 versus CCTA), with <3 mm leak in 21/102 (20.6%), 3-5 mm leak in 14/102 (13.7%), and ≥5 mm leak not observed. The incidence of PDL with the WATCHMAN was 29/79 (36.7%) and with the ACP/Amulet was 6/23 (26.1%) on TEE (p=0.46). All patients with TEE PDL also had CCTA patency. Among patients with PDL on CCTA, 67.3% also had PDL on TEE, with corresponding 100% sensitivity and 66.67% specificity. In terms of PDL size, there was moderate correlation between the leak size measured on CCTA versus TEE (r=0.484, p=0.007).
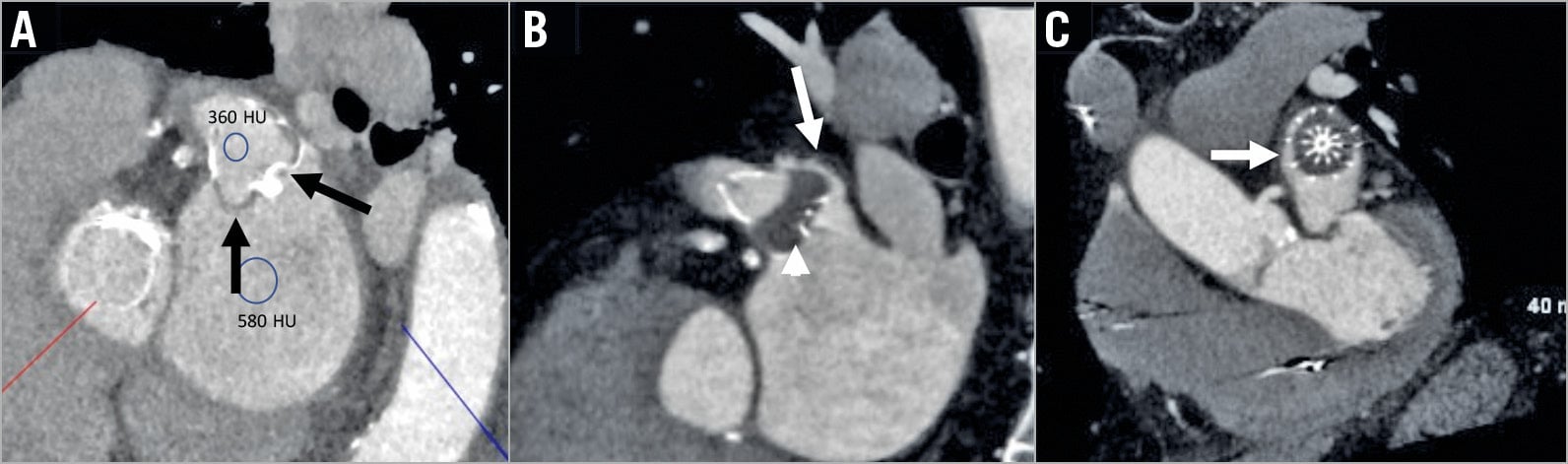
Figure 2. Contrast-enhanced CT. A) Fabric leak through an incompletely endothelialised WATCHMAN. Contrast seeps through the membrane at the proximal and distal shoulders of the device (black arrows) outlining the LAA. B) & C) WATCHMAN with contrast leaks through the postero-superior peri-device gap into the LAA (white arrows) with complete endothelialisation of the membrane (arrowhead).
The median linear attenuation coefficient in patients with LAA patency was 388 HU (133-670) with all patent LAA having >100 HU. The median linear attenuation coefficient in patients with an occluded LAA was 57 HU (16-89), with all sealed devices displaying attenuation <100 HU (p=0.001). The LA:LAA attenuation ratio was significantly higher in patients with patent devices (0.91), compared to patients with an occluded LAA (0.15) (p=0.001) (Figure 3). There were 3/48 (6.2%) patients with an occluded LAA with an LA:LAA ratio >0.25 (mean 0.29, but all values were <0.35).
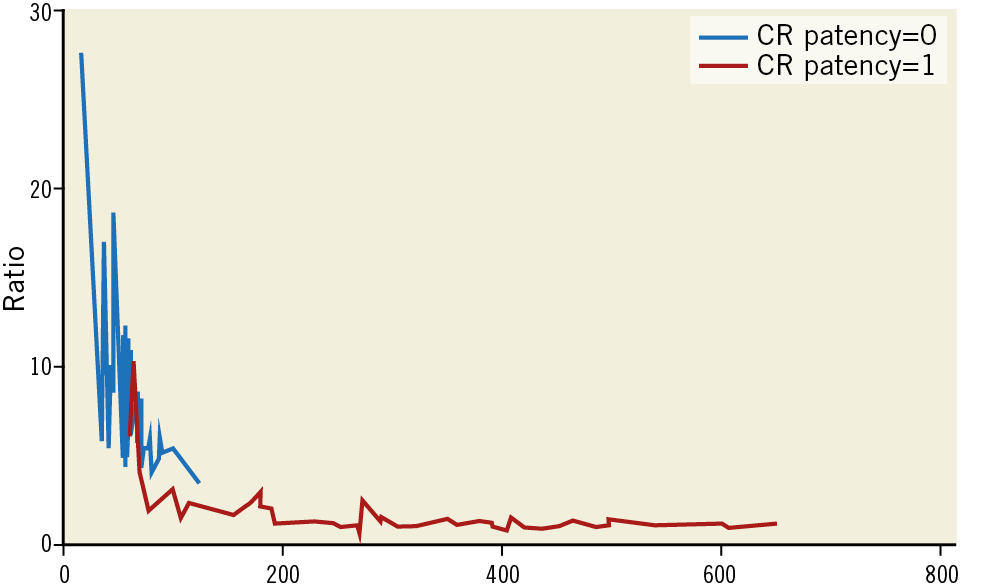
Figure 3. Linear attenuation coefficient (X-axis) versus LA:LAA attenuation ratio (Y-axis) in patent (blue line) and occluded (red line) LAA on post-implantation CCTA.
Patients with LAA patency on CCTA had greater mean device compression compared to those without residual patency (11.3±4.3% versus 8.2±4.0%, p<0.001) (Table 2), with no significant difference in other patient, anatomic or device characteristics. The mean device compression was 9.4±3.9% for patent WATCHMAN, and 11.3±4.1% (p=0.033) for sealed devices. Mean compression for ACP/Amulet was 5.4±3.1% with PDL and 11.2±5.7% without PDL (p=0.026). Multiplanar reconstruction on CCTA identified the PDL site predominantly at the posterior margin of the devices in 34/45 (75.5%) (Figure 2), with 32/34 (94.1%) leaks at the posterior-inferior margin (Supplementary Table 2).
CCTAs performed for ACP/Amulet were also assessed for the CLOSE criteria. The device lobe was tyre-shaped in all patients. Separation of at least 2 mm between the lobe and disc was seen in 18/23 (78.3%) patients. The disc was concave in 22/23 (95.6%). The lobe was implanted at least 2/3 deeper than the circumflex artery in 18/23 (78.3%). There were 5/23 (21.7%) cases where the lobes were off-axis at the landing zone on CCTA (Figure 4). However, only 1/23 (4.3%) appeared off-axis on procedural TEE, with the remainder, 22/23 (95.7%), deemed well-aligned. In 5/11 (45.4%) cases patent ACP/Amulet had an off-axis lobe, whereas none of the sealed ACP/Amulet had an off-axis lobe. Both Amulet devices implanted in the sandwich position (off-axis lobe) had patent LAA.
Figure 4. Post-LAAC CCTA with the Amulet. A) & B) Axial and sagittal images showing off-axis lobe (white arrow). C) Coronal image shows anterior-superior gap in LAA ostium with contrast leak (white arrow). D) Cine-rendered image showing the off-axis lobe of the Amulet.
A second post-implantation CCTA was performed in four patients with patent LAA. Of these, 3/4 were with ACP/Amulet that had persistent leak due to an off-axis lobe, and the median peri-ostial gap was 3 mm. One repeat CCTA with the WATCHMAN showed subsequent occluded LAA, with an initial leak mechanism due to fabric leak (first CCTA performed 57 days post LAAC).
DRT was seen in only one patient with the WATCHMAN on CCTA at 78 days post implant while on DAPT (planned DAPT for three months) (Figure 5), which was also confirmed on TEE. This patient was successfully treated with three months of warfarin with thrombus resolution and no clinical sequelae. This patient did not have PDL on CCTA/TEE. Two had clinically insignificant pericardial effusion on follow-up CCTA. There was no device embolisation or migration.
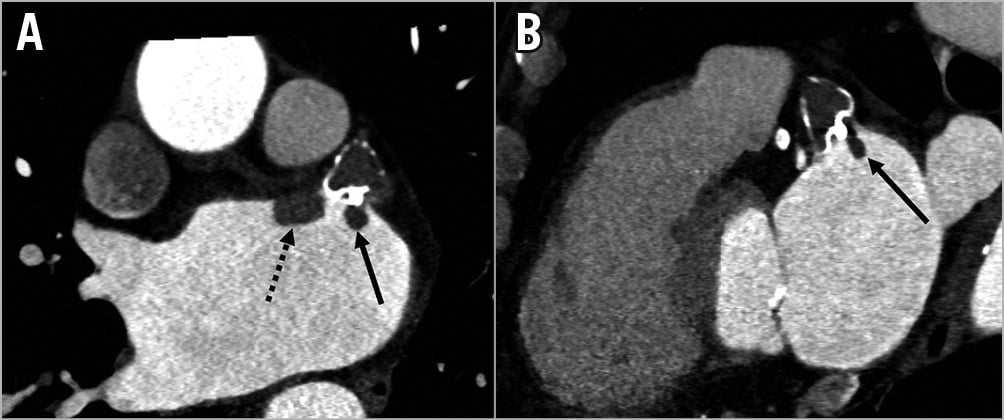
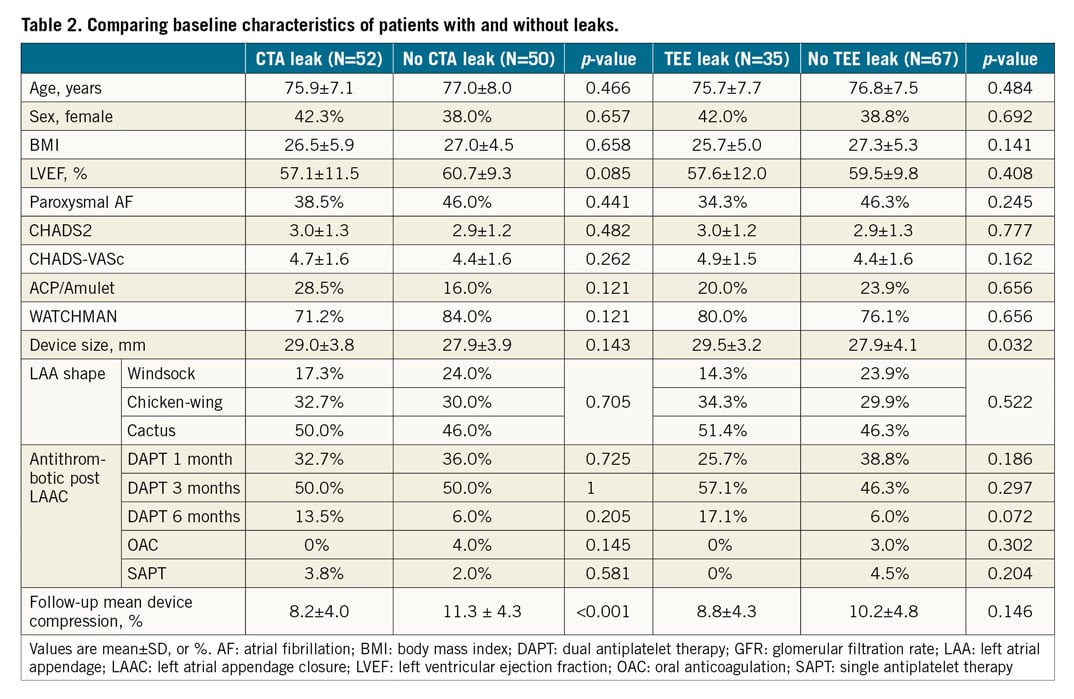
Figure 5. DRT post LAAC. Contrast-enhanced CCTA (A & B) shows atrial-side DRT in a patient with the WATCHMAN adjacent to the anterior aspect of the device (dotted arrow) and at the threaded fabric insert (full arrow).
The median duration of follow-up was 177 days (IQR 66-546), with no stroke or systemic embolism, or death. One patient had a transient ischaemic attack with no signs of cerebral infarction on imaging and did not have PDL or DRT. Patients with residual PDL did not have adverse clinical events.
Discussion
In our study comparing the utility of CCTA to TEE for assessing LAAC device-related complications, CCTA was superior for detecting residual LAA patency. In fact, CCTA detected a greater proportion with residual contrast in the remnant LAA, indicating greater sensitivity compared to TEE. In addition, the greater spatial resolution and 3D depiction of cardiac structures with CCTA allowed assessment of the mechanisms of LAA patency (such as fabric leak, lobe axis, and device compression). The presence of PDL was not associated with adverse clinical events during short-term follow-up in our patients.
Incomplete LAA seal is one of the major technical limitations of LAAC, either surgically or percutaneously. Incomplete LAAC results in an open pouch with residual flow into the LAA that may cause turbulent blood flow adjacent to the device, and stagnant blood in the remnant LAA. These may theoretically enhance platelet adhesion and thrombus formation that may then embolise to systemic circulation10. With surgical closures, incomplete closure increased the risk of LAA thrombus and thromboembolic events11,12. Fortunately, incomplete closures with percutaneous LAAC have not been shown to be associated with adverse events10,13. However, the primary method to date for assessing PDL has been TEE, which may not be the most sensitive technique for detecting incomplete closure, as was suggested in prior smaller case series5,14.
Our study is one of the largest to date to compare PDL on CCTA versus TEE, with prior published studies including <30 patients with concomitant CCTA and TEE post LAAC5,14. In our study, a greater proportion (52.0%) had residual LAA patency on CCTA compared to the proportion with PDL (35.0%) on TEE. CCTA was able to detect all TEE cases of PDL. In fact, it was more sensitive, being able to detect trivial leaks (≤1 mm) that were missed on TEE. However, the clinical significance of these trivial or even more substantial PDL on CCTA is unknown. Our study results are concordant with findings from Jaguszewski et al, where 62% and 36% patent LAA were identified on CCTA and TEE, respectively14.
A small proportion (13.5%) of those with residual patency on CCTA was due to fabric leak (i.e., diffusion of contrast through membrane). This mechanism of leak was not detected on TEE. All cases of fabric leak were with the WATCHMAN (covered by permeable 160 µm membrane): these were well-aligned and situated appropriately at the LAA ostium. The presumed mechanism for fabric leak is incomplete endothelialisation of the device surface, the timing of which can vary significantly between patients. Complete WATCHMAN endothelialisation was shown to occur in a canine model at ~28-45 days15,16; however, the timing in humans may be variable or delayed. The risk of thrombus formation in the LAA and systemic embolisation can presumably occur in the presence of fabric leak, similar to PDL. Therefore, it is relevant to detect these different mechanisms of incomplete LAAC.
The degree of contrast opacification distal to the LAAC device can be subjectively assessed or be quantitated objectively by measuring the linear attenuation coefficient. We previously showed that a cut-off of 100 HU within the LAA can differentiate between a sealed or patent device5. Additionally, a ratio of LAA and LA attenuation coefficient of <0.25 was shown by Homsi et al to validate a sealed device17. Our study results support both these cut-off values. All completely occluded LAA post LAAC had attenuation <100 HU (Figure 1). There were 27/30 (90%) occluded LAA with an LAA:LA ratio of <0.25. The remainder, 3/30 (10%), of occluded LAA had an LAA:LA attenuation ratio of between 0.25 and 0.35, with a mean value of 0.29. These values can be affected by technical differences during image acquisition leading to different atrial contrast attenuation, thus altering the ratio.
CCTA can help to delineate the mechanisms of LAA patency. Adequate sizing and positioning of the LAAC device at the LAA ostium is imperative for successful closure8. We found that CCTA can show the shape and position of the device well, providing detailed information on the device axis relative to cardiac structure orientation, and with far better spatial resolution compared to TEE. We were able to assess the location accurately and measure the size of the PDL. We identified the posterior quadrant to be the predominant site of PDL. It is not clear why most PDL occurred in the postero-inferior quadrant. This may be related to the elliptical shape of the LAA ostia with the long axis in the postero-inferior to antero-superior direction, and therefore a greater gap along the long-axis direction. We found that occluded LAAs had significantly higher mean device compression compared to patients with patent LAAs, suggesting that higher device compression reduces residual leak. This confirmed findings from earlier smaller series5,14. We did not find other differences in patient, anatomic or device characteristics that affected LAA patency.
We also assessed the proper deployment criteria for the ACP/Amulet. CCTA identified 41.6% off-axis ACP/Amulet devices, compared to only 8.3% malaligned devices on TEE. These results highlight the limited 3D evaluation of LAA morphology and device position on TEE. Prior studies had shown that LAA patency was seen in patients with suboptimal lobe alignment leading to contrast diffusion through the edges of a malapposed lobe at the landing zone5,18. Of note, all ACP/Amulet implanted using the sandwich technique had patent LAA, which could be explained by lack of apposition of the lobe against the appendage wall, allowing contrast to fill through the edges of the disc and lobe (Figure 6). Since having a properly aligned device with the LAA wall appears important to minimise PDL, having steerable sheaths to improve device alignment during implant may be useful.
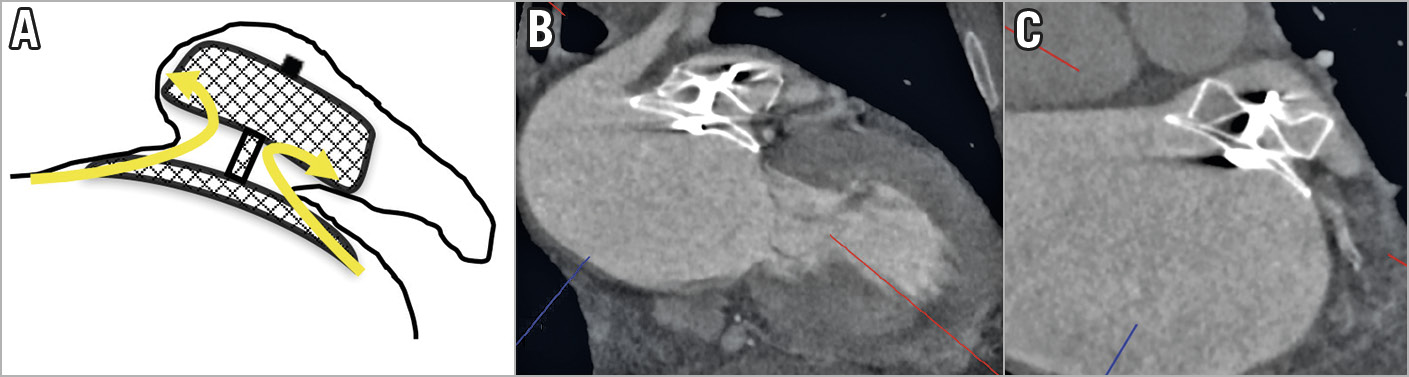
Figure 6. Sandwich implant technique with an Amulet resulting in residual LAA contrast opacification. A) Schematic diagram showing contrast leaking through the sides of the Amulet lobe (arrows). B) & C) CCTA oblique images showing off-axis lobe from the sandwich technique resulting in LAA contrast opacification.
Although CCTA is a good non-invasive alternative to TEE after LAAC, there are limitations such as cost, radiation dose, and exclusion in patients with severe renal dysfunction. According to the American College of Radiology guidelines, patients with a GFR >60 ml/min/1.73 m2 are at very low risk for contrast-induced nephropathy. For patients with stable baseline GFR 30-45 ml/min/1.73 m2, intravenous contrast media is considered a moderate nephrotoxic risk factor, and oral or IV hydration is recommended. Patients with unstable renal function and GFR <30 ml/min/1.73 m2 are particularly at risk for developing contrast-induced nephropathy19, and thus TEE will remain the investigation of choice for surveillance.
Limitations
This is a small retrospective study with low clinical event rates, although it provides some of the largest data to date comparing CCTA to TEE after LAAC. CCTA identified a greater proportion of patients with PDL; however, the clinical significance compared to TEE of this identified leak is unclear. Larger combined multicentre CCTA series should be explored to assess whether the presence of LAA patency correlates to adverse long-term clinical events.
Conclusions
CCTA is a suitable non-invasive alternative to TEE after endovascular LAAC for assessment of device success and complications, including PDL and DRT. CCTA identified more residual LAA patency than TEE, with the majority due to PDL. Thus, CCTA appears to be superior to TEE in assessing PDL and the mechanisms of leak. A lower degree of device compression and mal-aligned devices was associated with a greater incidence of PDL.
|
Impact on daily practice Routine LAAC device surveillance is important to assess for PDL and DRT. TEE is the standard method used; however, it is invasive and is less sensitive than CCTA to assess for PDL. Furthermore, CCTA can evaluate the mechanism of PDL. Our study shows CCTA to be a feasible alternative to TEE for device surveillance post LAAC. |
Conflict of interest statement
J. Saw reports grants from the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Canada, the National Institutes of Health, AstraZeneca, Abbott Vascular, Servier, and St. Jude Medical, during the conduct of the study. The other authors have no conflicts of interest to declare.

