Despite the fact that percutaneous left atrial appendage (LAA) closure has been performed for more than ten years1,2, there has only been little – and hesitant – evolution in the way we prepare for this procedure. As the LAA anatomy is highly variable and complex, accurate assessment of its structure is essential for a safe and successful procedure. Traditionally, imaging and sizing of the LAA has relied on transoesophageal echocardiography (TEE). However, in parallel with the acceptance of cardiac computed tomography angiography (CCTA) as the “gold standard” imaging tool to prepare for transcatheter aortic valve implantation (TAVI), nowadays CCTA is also increasingly recognised as a valuable preprocedural imaging modality to prepare for percutaneous LAA closure. This editorial describes some of the options and benefits of using CCTA when preparing for percutaneous LAA closure (Figure 1).
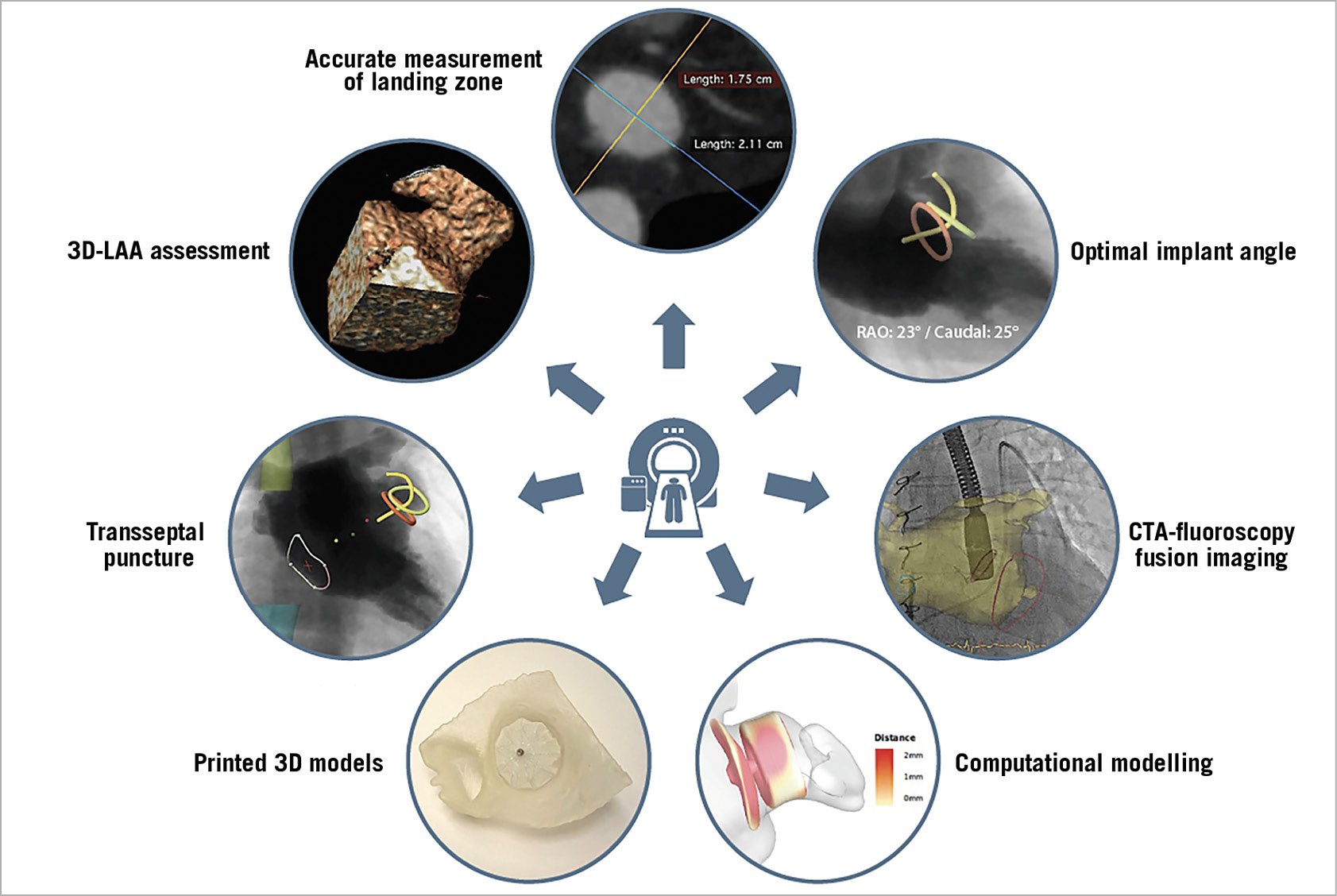
Figure 1. Possible applications of cardiac computed tomography angiography (CCTA), which can contribute to a better preparation of percutaneous LAA closure.
Three-dimensional (3D) LAA assessment
As the LAA is a complex 3D structure often with multiple lobes in different planes, a thorough and accurate 3D assessment of this cardiac structure helps in obtaining a solid preprocedural plan. Not only the number of LAA lobes but also the orientation of the lobe(s) may have an impact on the preferred LAA closure device, device positioning (e.g., distal end of the device in the superior lobe) and even on the preferred location for transseptal puncture. Whereas 3D visualisation of CCTA data using volume rendering is a standard and easy option in all CCTA analysis software packages3,4, this information can be more difficult to obtain by and interpret on TEE imaging. An additional advantage for the operator performing the LAA closure procedure is that 3D volume-rendered CCTA images are much easier to compare and link to the fluoroscopic images obtained during the intervention.
Accurate measurement of the LAA landing zone
Although official instructions for use and sizing charts for LAA closure devices are (still) based on two-dimensional (2D)-TEE imaging, this methodology has its shortcomings. As the LAA is most often an elliptical structure, accurate measurement of the maximum diameter (or perimeter) of the LAA ostium and LAA landing zone should be made on 3D double-oblique images, which can be easily provided by CCTA-based 3D multiplanar reconstruction. This may not be possible on 2D-TEE images5. The same rationale is valid for exact measurement of the elliptical aortic annulus by 2D-TEE vs. CCTA in order to prepare for TAVI6. The use of 3D-TEE imaging could theoretically overcome this limitation; however, determining and measuring the LAA landing zone by 3D-TEE may often not be feasible. In addition, measurement of the LAA depth (even in different lobes) and assessment of LAA trabecularisation and/or thrombus are feasible and accurate on CCTA imaging.
Optimal implant angle
Besides measurement of LAA dimensions, CCTA analysis also allows predicting the optimal C-arm angle for LAA closure device implantation. First, the C-arm angle in which the LAA central axis will be the least foreshortened can be estimated. This can be useful when deep intubation of the LAA is needed. Second, and more importantly, the C-arm angle in which the LAA ostium and/or landing zone will be aligned can be predicted. This latter C-arm angle is not only the best projection to assess device compression but is also helpful in verifying coaxial alignment of the LAA closure device with the LAA structure. This is of importance, as off-axis LAA closure device implantation has been reported to be associated with a higher risk of peri-device leakage7. Moreover, as this CCTA analysis to determine the optimal C-arm implant angle(s) can be made “off-line” – before the actual procedure – applying this methodology can result in less radiation, less use of contrast dye and potentially even reduce procedural complications.
Optimal location for the transseptal puncture
The location of the transseptal puncture site impacts on the possibility of obtaining a coaxial orientation of the delivery sheath with the LAA and, hence, the procedural complexity and outcome. Determining the optimal transseptal puncture site on CCTA is possible and is dependent on the LAA position and orientation. In the early days of percutaneous LAA closure, a standard inferoposterior puncture was recommended for all cases8. However, a more anterior transseptal puncture should sometimes be considered in case of a more posteriorly oriented LAA lobe. This can be assessed and detected on preprocedural CCTA. Also, the location of other cardiac structures, such as the aortic valve, relative to the optimal transseptal puncture site can be determined on CCTA. This can be helpful when performing the LAA closure procedure without TEE guidance. In such cases, it can be helpful to have a radial transarterial pigtail just above the aortic valve in order to guide the transseptal puncture9.
Possibility of CCTA-fluoroscopy fusion imaging
The use of fusion imaging in complex structural heart interventions has gained increasing interest over the past decade. Currently, only the combination of TEE with fluoroscopy allows real-time fusion imaging. The shortcoming of CCTA and fluoroscopy overlay is the inability of using live fusion. Still, CCTA-fluoroscopy fusion imaging has shown its value by providing visual anatomical markers during ablation procedures10. The use of markers can also be helpful to localise the otherwise invisible LAA on fluoroscopy and can potentially increase procedural success while reducing radiation dose, procedure time, and the use of contrast dye. Markers can be placed at the LAA orifice, LAA landing zone, or the tip of the LAA. In addition, overlay imaging may help with the LAA orientation and ensure correct device positioning.
Printed 3D models
Although CCTA allows better understanding and sizing of the patient’s LAA anatomy, predicting the actual landing zone of the LAA closure device still remains difficult and an important source of sizing error. When using patient-specific CCTA-based 3D LAA models, one can test different LAA closure device sizes at different implant depths and/or orientations. In addition, the device compression rate and even leakage of small beads can be tested. In a pilot study using printed 3D models, the number of LAA closure devices used per procedure was significantly lower in those cases prepared with 3D model testing as compared to CT- or TEE-sizing only (1.05, 1.2 and 1.4 devices per procedure, respectively). Also the number of cases with remaining contrast leakage into the LAA at CCTA follow-up was only 1/20 for the CCTA-3D model group as compared to 4/20 for the CCTA-only group11. Although the importance of complete LAA closure is still a topic of debate, it seems obvious that complete LAA closure should be preferred over remaining peri-device leaks.
Computational modelling
In accordance with 3D model testing, computational modelling can provide additional insights into patient-specific LAA anatomy and its interaction with the implanted device. Dedicated software has been developed to simulate different types and sizes of LAA closure device at different implant depths within a patient-specific LAA anatomy. The computational model also generates information on device compression and wall apposition, the latter being predictive for the risk of LAA leakage (data not published yet). An additional advantage of computational modelling is that any device can be tested at any moment. When using 3D models, these models need to printed, which is time-consuming, and an entire bench-test set of LAA occluders has to be available. These limitations do not apply to computational modelling. A randomised controlled trial comparing standard planning with computational model-assisted planning of LAA closure procedures will start mid 2019.
Possibility of performing LAA closure under local anaesthesia
Since the use of CCTA allows comprehensive and accurate preprocedural planning, several centres are nowadays performing percutaneous LAA closures under local anaesthesia. In order to guide the critical steps of LAA closure and evaluate the implant result during such procedures under local anaesthesia, most operators have been using intracardiac echocardiography (ICE), which can be introduced via the femoral vein and introduced into the left atrium12. Other operators are more familiar with micro-TEE for guidance of percutaneous LAA closure. However, as both ICE and micro-TEE have their limitations, especially for accurate sizing of the LAA, it seems essential that such an approach should only be chosen when high-quality CCTA imaging is available preprocedurally. As general anaesthesia is no longer an absolute need for performing LAA closure, this approach may also facilitate the entire logistical process in some hospitals.
In conclusion, it may not be surprising if CCTA increasingly replaces TEE as the preferred imaging tool to prepare for percutaneous LAA closure. Those operators already using preprocedural CCTA to plan LAA closure are not willing to return to a “TEE-only” approach. However, more data supporting these advantages of CCTA are needed and CCTA-based instructions for use from the LAA closure device vendors will have to follow in order to establish CCTA as the new “gold standard” imaging tool to prepare for percutaneous LAA closure.
Conflict of interest statement
The authors have no conflicts of interest to declare.

