Abstract
Aims: To assess the left atrial appendage (LAA) geometry with multidetector-row computed tomography (MDCT) and its implications for selection of closure devices.
Methods and results: One hundred and ninety-seven patients who underwent MDCT prior to catheter ablation for atrial fibrillation were evaluated. Feasibility for Watchman and Amplatzer Cardiac Plug (ACP) devices was assessed based on the maximal cross-sectional diameter and perimeter of the ostium and at 10 mm depth and on the LAA diameter on the MDCT plane resembling the transoesophageal echocardiography (TEE) view. Mean maximal diameters of the ostium and at 10 mm depth were 28.7±4.4 mm and 24.6±4.5 mm, respectively, resulting in feasibilities of 80.7%, 84.8% and 91.4% for the Watchman, the ACP and for either one of the two devices, respectively. Mean perimeters of the ostium and at 10 mm depth were 79.1±12.2 mm and 69.8±11.6 mm, resulting in feasibilities of 87.8%, 92.9% and 96.4% for the Watchman, the ACP and for either one of the two devices, respectively. Mean TEE-like MDCT LAA diameter was 22.0±3.3 mm, resulting in feasibilities of 93.9%, 97% and 99.0% for the Watchman, the ACP and for either one of the two devices, respectively.
Conclusions: The feasibility of current devices is high, based on MDCT measurements of the LAA, with no difference for either one of the devices.
Introduction
Atrial fibrillation (AF) is a common cardiac arrhythmia associated with >20% of the strokes in patients aged >80 years1,2. Oral anticoagulant drug therapy is a well-established effective treatment in preventing thromboembolic events and is the treatment of first choice in this population3. However, vitamin K antagonists (warfarin) have a narrow therapeutic window, many drug-drug and drug-food interactions, and are associated with an increased risk of bleeding, resulting in a limited use of this therapy in high-risk patients4. Moreover, long-term compliance remains problematic as the Swedish Stroke Register demonstrated: a decline in use to 45% after two years5. Bearing in mind that in non-valvular AF the left atrial appendage (LAA) is the main source of thrombi6, the PROTECT AF trial was designed to demonstrate the efficacy of an LAA occluder device in stroke prophylaxis7. Despite transcatheter LAA occlusion being non-inferior to warfarin for preventing stroke, the significant rates of periprocedural complications, including device embolisation and pericardial effusion, led to safety concerns8. Accurate sizing of the implanted device and accurate procedural manipulation of catheters and delivery systems may improve the results of this therapy and minimise the number of complications.
The LAA is a complex and heterogeneous structure with a rather elliptical ostium, which may challenge the sizing of the occluder device with two-dimensional (2D) imaging modalities9-11. Multidetector-row computed tomography (MDCT) provides high spatial resolution three-dimensional (3D) data of the LAA and may help to select a more appropriate size of LAA occluder device. Accordingly, the aim of the present study was to describe the LAA morphology and dimensions of non-valvular AF patients who underwent MDCT prior to radiofrequency catheter ablation. The preprocedural feasibility for current LAA occluder devices based on MDCT measurements was assessed.
Methods
PATIENTS
A total of 197 patients who underwent MDCT prior to radiofrequency catheter ablation for symptomatic, drug-refractory, non-valvular AF were evaluated. MDCT was performed to guide the ablation procedure with a detailed visualisation of the anatomy and dimensions of the left atrium and pulmonary veins and to exclude the presence of significant coronary artery disease. Patients with an adequate and complete visualisation of the LAA on the MDCT images were included. Clinical and MDCT data were collected in the departmental electronic clinical files (EPD Vision, version 8.3.3.6; Leiden, The Netherlands) and retrospectively analysed.
MULTIDETECTOR-ROW COMPUTED TOMOGRAPHY DATA ACQUISITION
MDCT data were acquired with either a 64-detector-row computed tomography scanner (Toshiba Multislice Aquilion 64; Toshiba Medical Systems, Otawara, Japan) or a volumetric 320-detector-row computed tomography scanner (Aquilion ONE; Toshiba Medical Systems, Tochigi-ken, Japan). For the Aquilion 64 the rotation time was 400 msec and a collimation of 64×0.5 mm was set. Depending on the body mass index of the patients, a tube voltage between 100 and 135 kV and a tube current of 250-400 mA was chosen. For the Aquilion ONE the rotation time was 350 msec, the collimation was set at 320×0.5 mm and the tube voltages and currents were 100-135 kV and 400-580 mA, respectively. Beta-blockers or ivabradine were administered to patients with heart rates >65 beats per minute (bpm), unless clinically contraindicated.
The volume of non-ionic contrast media (Iomeron 400; Bracco, Milan, Italy) was administered in the antecubital vein depending on the body weight, total scan time and renal function. For the Aquilion 64 system the administration flow rate was 5 mL/s and the total amount varied from 80 to 110 mL. With the Aquilion ONE system, 60-100 mL of contrast media was administered in three consecutive steps: first, 50-90 mL of contrast media was infused at a flow rate of 5.0-6.0 mL/s, followed by a 20 mL mixture of 50% contrast/saline, which again was followed by the infusion of 25 mL saline at a flow rate of 3.0 mL/s.
For synchronisation of the contrast media arrival and the beginning of the scan, automated peak enhancement detection in the left ventricle was used for the detection of the contrast bolus and, after reaching +180 HU, craniocaudal scanning was initiated. Image acquisition was performed during an inspiratory breath-hold of 8 to 10 seconds. For the Aquilion 64 system the electrocardiogram (ECG) was simultaneously recorded for retrospective gating. Image reconstruction was performed at both 30-35% and 75-85% phases of the RR interval for the systole and diastole, respectively. With the Aquilion ONE system, prospective ECG triggered dose modulation was used to visualise an entire cardiac cycle with the accomplishment of maximal tube current at 75%, 65-85% or 30-80% of the RR interval in patients with heart rates of <60 bpm, 60-65 bpm or >65 bpm, respectively. Beyond these intervals, the tube current was only 25% of the maximal tube current. The mean effective dose of the 177 CTs acquired on the 320-row system was 3.9±1.8 mSv. The mean radiation dose for 64-slice CT (n=20) has been previously described and was 18.1±5.9 mSv12.
MDCT data were reconstructed with a slice thickness of 0.5 mm and with a reconstruction interval of 0.3 mm and 0.25 mm for the Aquilion 64 and Aquilion ONE systems, respectively, and transferred to a workstation for further post-processing and analysis off-line (Vitrea 2; Vital Images, Plymouth, MN, USA).
MULTIDETECTOR-ROW COMPUTED TOMOGRAPHY DATA ANALYSIS
LAA MORPHOLOGY
For assessment of the LAA morphology, 3D volume-rendered images were used and the LAA morphologies were categorised into four different types. The LAA shape was classified as “windsock” if the primary structure was one dominant lobe with sufficient length, as “chicken wing” if there was an obvious bend in the proximal or middle part of the dominant lobe, as “cauliflower” for an LAA with limited length and a distal width exceeding the proximal width, and as “swan” if the LAA presented a second sharp curve folding the structure back (Figure 1)13,14.
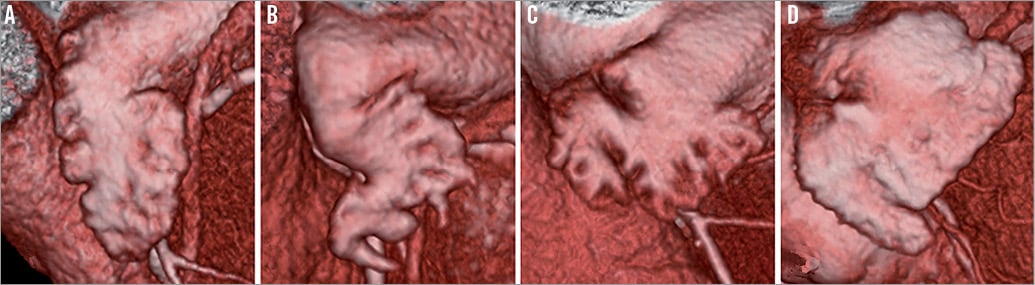
Figure 1. Morphologies of the LAA. The LAA morphology was classified as a windsock (A), chicken wing (B), cauliflower (C) or swan (D).
LAA OSTIUM AND NECK DIMENSIONS
A double oblique sagittal view was reconstructed with orientation of both the orthogonal axial and the single oblique coronal views to measure the dimensions of the ostium and neck of the LAA in a cross-sectional plane parallel to the LAA ostium. From this view, the cross-sectional minimal and maximal diameter, perimeter and area of the ostium were measured (Figure 2). Thereafter, the axial view was oriented 10 mm deeper into the LAA along its long axis and at this point a new double oblique sagittal view of the LAA neck was reconstructed and the above-mentioned variables were also measured.
ANGULATION OF THE LAA, LAA LENGTH AND DISTANCE FROM THE OSTIUM TO THE FIRST BEND OF THE LAA
The angle of the LAA with reference to the ostium, the length of the LAA from its ostium to its apex and the distance from the LAA ostium to its first bend were analysed in a single oblique coronal view reconstructed by orientation of the orthogonal axial view (Figure 2).
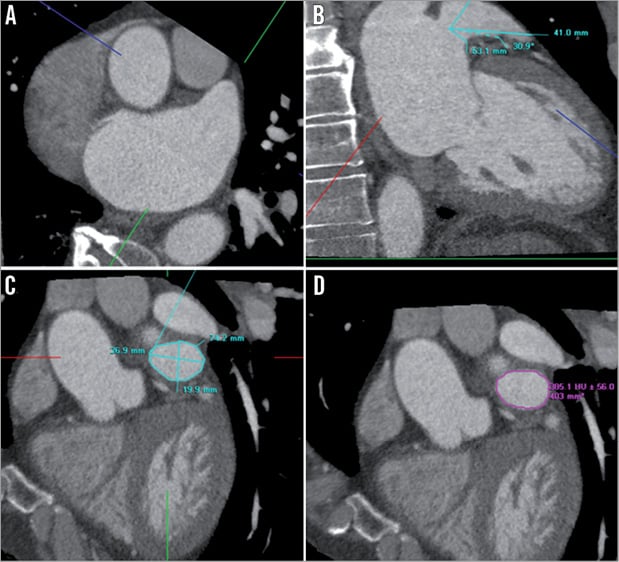
Figure 2. Anatomical analysis of the left atrial appendage. The orthogonal axial (A) and the single oblique coronal views (B) were used to reconstruct a double oblique sagittal view parallel to the LAA ostium (C and D). The angulation of the LAA with reference to the ostium, the LAA length and the distance from ostium to the first bend of the LAA were measured in the single oblique coronal view and the cross-sectional minimal and maximal diameter, perimeter and area were measured in the double oblique sagittal view.
DISTANCE FROM THE FORAMEN OVALE TO THE LAA OSTIUM
The distance between the foramen ovale and the LAA ostium was measured in a single oblique coronal view reconstructed with the orthogonal axial and sagittal views for orientation (Figure 3).
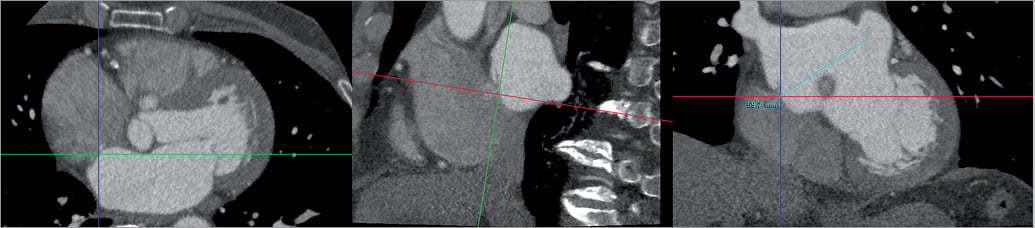
Figure 3. Foramen ovale to LAA ostium distance. The distance between the foramen ovale and the LAA ostium was assessed in a single oblique coronal view reconstructed with the axial and sagittal views.
LAA DIAMETER ACCORDING TO THE TRANSOESOPHAGEAL ECHOCARDIOGRAPHY VIEW
Currently, LAA occlusion devices are sized on the maximal LAA ostium diameter measured from the mid-oesophageal 45-90º transoesophageal echocardiography (TEE) view. From MDCT data, the mid-oesophageal 45-90° TEE view was reconstructed and the LAA diameter was measured in a plane between the left circumflex artery and the LAA wall 10 mm below the ridge of the left superior pulmonary vein (LSPV) or limbus (Figure 4)15,16.
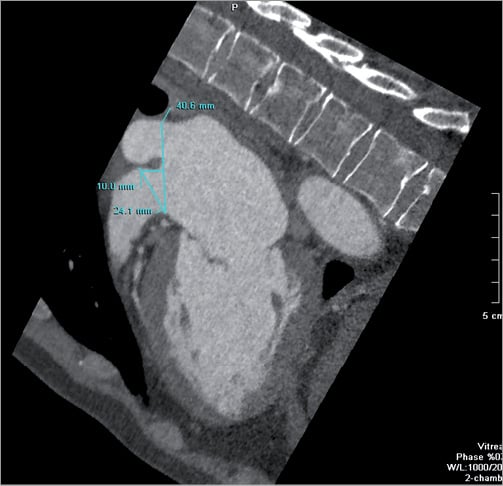
Figure 4. LAA diameter according to mid-oesophageal 45-90° TEE view. The mid-oesophageal 45-90° view was reconstructed to measure the LAA diameter in a plane between the left circumflex artery and the LAA wall 10 mm below the ridge of the left superior pulmonary vein or limbus.
LAA CLOSURE DEVICES: FEASIBILITY
The feasibility for current LAA occlusion devices was assessed based on three different sizing approaches: the maximal cross-sectional diameter and perimeter and on the TEE-like MDCT LAA diameter.
WATCHMAN DEVICE
The Watchman device (Atritech Inc., Minneapolis, MN, USA) is a self-expandable nitinol frame structure with fixation barbs and polyester fabric available in diameters from 21 to 33 mm, accommodating maximal LAA ostium diameters between 17 and 32 mm7,8. In addition, based on the nominal device diameters, the lower and upper limits of the device perimeter were derived, ranging from 66.0 mm to 103.7 mm, respectively. Finally, the length of the LAA should also be measured to ensure a landing zone that exceeds the maximal ostium diameter.
AMPLATZER CARDIAC PLUG
The Amplatzer Cardiac Plug (ACP) (AGA Medical Corporation, North Plymouth, MN, USA) consists of two mesh nitinol bodies: a distal anchoring lobe and a proximal sealing disc linked via a compliant waist. The distal lobe is available in several diameters ranging from 16 to 30 mm. According to the manufacturer’s recommendations, the LAA diameter should be measured 10 mm deep from the LA wall (the most proximal zone to let the Amplatzer Cardiac Plug land for a stable fixation). In addition, the distal lobe should be 1.5 to 3.4 mm larger than that diameter, covering diameters from 12.6 to 28.5 mm17,18. This range was used for feasibility definition of both the maximal cross-sectional diameter at 10 mm depth and of the TEE-like MDCT LAA diameter. The lower and upper limits of the nominal perimeter derived from the distal lobe diameter were 50.3 mm and 94.2 mm, respectively.
STATISTICAL ANALYSIS
Continuous variables were evaluated for a normal distribution with the Kolmogorov-Smirnov test and are presented as mean±standard deviation. Categorical variables are presented as frequencies and percentages. All the statistical analyses were performed with SPSS software, version 20.0 (IBM Corp., Armonk, NY, USA).
Results
PATIENT CHARACTERISTICS
The clinical characteristics of the 197 patients (58±9 years, 153 [78%] male) with an adequate and complete visualisation of the LAA on MDCT are presented in Table 1. Reasons for insufficient quality were a too narrow scanning box for complete visualisation of the LAA (n=6), insufficient contrast medium in the LAA (n=2), and poor image quality due to an imbalance between tube current and tube voltage and the patient’s body mass index (n=5). No patients were excluded because of uninterpretable images due to irregular heart rhythm.
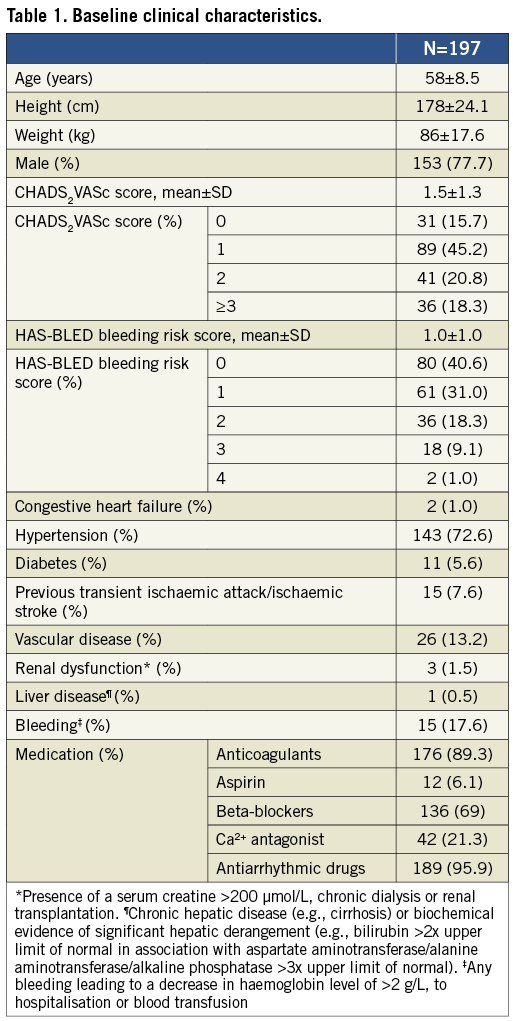
LAA MDCT measurements
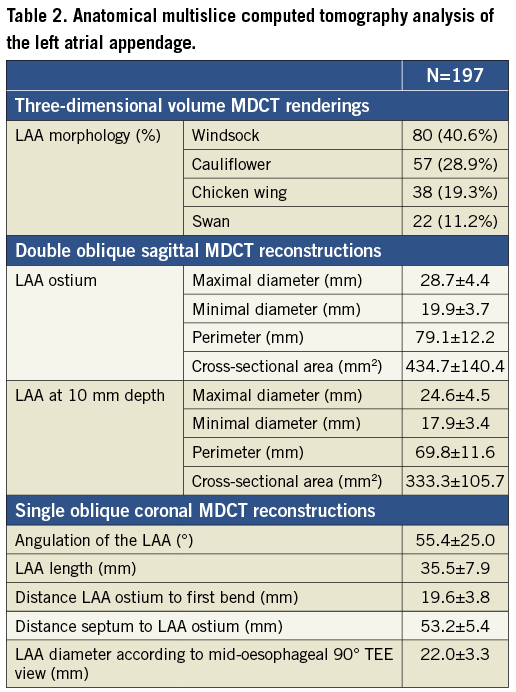
The results of the MDCT data analysis are summarised in Table 2.
LAA MORPHOLOGY
In 80 (41%) patients the morphology of the LAA was a windsock and in 57 (29%), 38 (19%) and 22 (11%) patients the morphologies were a cauliflower, chicken wing and swan, respectively.
LAA OSTIUM AND NECK DIMENSIONS
The mean minimal and maximal diameters of the LAA ostium were 19.9±3.7 mm and 28.7±4.4 mm, respectively. In the same plane, the mean perimeter was 79.1±12.2 mm and the mean cross-sectional area was 434.7±140.4 mm2. For the ACP device, these dimensions were re-measured at 10 mm depth in the LAA resulting in minimal and maximal diameters, perimeter and cross-sectional area of 17.9±3.4 mm, 24.6±4.5 mm, 69.8±11.6 mm and 333.3±105.7 mm2, respectively.
ANGULATION OF THE LAA, LAA LENGTH, DISTANCE FROM THE OSTIUM TO THE FIRST BEND OF THE LAA AND DISTANCE BETWEEN FORAMEN OVALE AND LAA OSTIUM
The mean angle of the LAA with reference to the ostium was 55.4±25.0°, the maximal LAA length was 35.5±7.9 mm and the distances from the LAA ostium to its first bend and from the foramen ovale to the LAA ostium were 19.6±3.8 mm and 53.2±5.4 mm, respectively.
LAA DIAMETER ACCORDING TO THE TEE VIEW
On the simulated reconstruction of the mid-oesophageal 45-90° TEE view, the mean LAA diameter measured 10 mm from the LSPV limbus was 22.0±3.3 mm. This resulted in an underestimation of 2.6 mm compared with the cross-sectional maximal diameter measured at 10 mm depth.
FEASIBILITY FOR LAA CLOSURE DEVICES ACCORDING TO MDCT MEASUREMENTS
The feasibility of the Watchman and the ACP devices based on MDCT measurements of the LAA are presented in Figure 5.
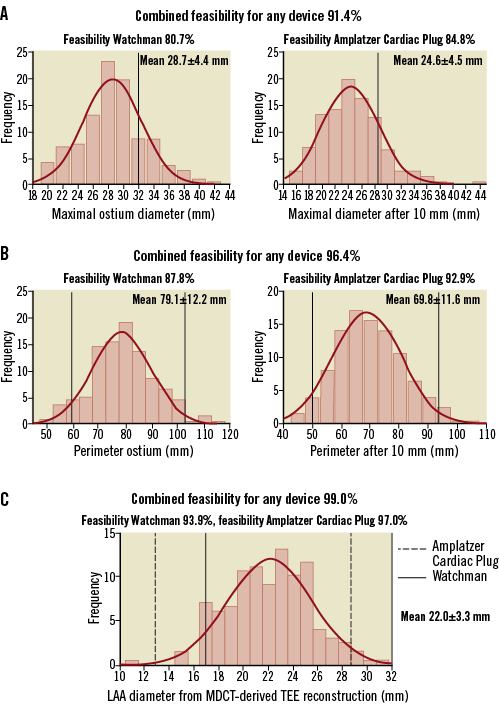
Figure 5. Feasibility for LAA closure devices according to MDCT measurements. Feasibility for both LAA closure devices was assessed with the maximal cross-sectional diameters (A), perimeters (B) and LAA diameters according to manufacturers’ recommendations. Black (solid and dotted) lines indicate the upper and lower sizes of the available devices.
WATCHMAN
Based on the maximal cross-sectional diameter of the ostium and the TEE-like MDCT LAA diameter, 159 (80.7%) and 185 (93.9%) patients would accommodate a Watchman device. Applying also the second requirement (the LAA length should exceed the maximal ostium diameter), a total of 154 (78.2%) and 182 (92.4%) patients would be suitable for LAA occlusion with the Watchman based on the maximal cross-sectional ostium diameter and the TEE-like MDCT diameter, respectively.
Based on the nominal perimeter of the device, the procedure would be feasible with this device in 173 (87.8%) patients. Finally, taking into consideration that the maximal ostium diameter should not be larger than the maximal LAA length, a total of 168 (85.3%) patients would be eligible for the Watchman device.
AMPLATZER CARDIAC PLUG
Based on the maximal cross-sectional diameter at 10 mm depth and the TEE-like MDCT LAA diameter, 167 (84.8%) and 191 (97.0%) patients would accommodate an ACP device. Based on the nominal perimeter of the device, the procedure would be feasible with this device in 183 (92.9%) patients.
COMBINED DEVICE FEASIBILITY
Based on the maximal cross-sectional diameter, a total of 180 (91.4%) patients would be suitable candidates for transcatheter LAA occlusion with either the Watchman or the ACP device. For the TEE-like MDCT LAA diameter and the nominal perimeter these overall feasibilities were, respectively, 195 (99.0%) and 190 (96.4%) patients.
Discussion
The present study demonstrated that MDCT can provide an accurate assessment of the LAA geometry in non-valvular AF patients. The ACP and the Watchman devices cover a broad spectrum of LAA morphologies and geometries resulting in a high procedural feasibility based on MDCT measurements of the LAA.
MULTIDETECTOR-ROW COMPUTED TOMOGRAPHY BEFORE PERCUTANEOUS LAA OCCLUSION
After demonstration of the efficacy of transcatheter LAA occlusion for stroke prophylaxis in non-valvular AF patients in the randomised PROTECT AF trial, the number of patients undergoing this procedure has grown significantly7,8,17-19. However, despite increased operator experience, the procedure remains associated with relatively high rates of periprocedural complications (device embolisation and pericardial effusion)7,8,19. Accurate sizing of the LAA ostium and a thorough knowledge of the LAA anatomy may reduce the frequency of these complications. Therefore, the use of imaging techniques for selection of patients who are candidates for these procedures is crucial.
Two-dimensional TEE remains the imaging technique of first choice to evaluate patients prior to transcatheter LAA closure7,8,17,18. However, this imaging modality assumes a circular shape of the LAA ostium and does not provide accurate visualisation of the cross-sectional plane of the LAA ostium and the landing zone which may result in underestimation of the LAA dimensions. In addition, it has been demonstrated that the dimensions of successfully implanted devices were 20-40% larger than those predicted by 2D TEE20. During the first experiences, periprocedural resizing of the LAA ostium and landing zone to select the device size was needed up to four times17,21. Data of the initial European experience with the Amplatzer Cardiac Plug showed that in 17% of the successfully implanted devices a second or third device was needed17.
In contrast, 3D imaging techniques provide volume renderings of the LAA that can be further cropped with the use of multiplanar reformation planes to obtain the true maximum diameter of the LAA at the precise level where the device will be anchored.
Three-dimensional TEE permits accurate measurement of the LAA dimensions during the procedure and does not need the use of iodinated contrast, which may be a relative contraindication in patients with renal dysfunction. However, with 3D TEE, Nucifora et al demonstrated that the LAA ostium area was underestimated when compared with MDCT11. Most likely this is due to the higher spatial resolution of MDCT.
The present evaluation provides interesting data in this field by comparing the device feasibility according to the maximum cross-sectional LAA diameter and perimeter measured on MDCT and to the LAA diameter measured on a MDCT TEE-like projection. The TEE-like MDCT LAA diameter was 2.6 mm smaller than the maximal cross-sectional diameter at 10 mm depth. This may explain the higher procedural feasibility using the TEE-like MDCT LAA diameter as compared with the maximum cross-sectional LAA diameter, since this last measurement was larger than the maximum nominal device diameter in a significant percentage of patients (19.3%).
Using the maximal cross-sectional diameter for sizing the LAA it is important to realise that the shape of the LAA ostium will conform to the implanted, self-expanding occluder. In particular, this will result in a change in diameter rather than in the perimeter. Bearing this in mind, a sizing approach based on the planimetered perimeter may represent the most accurate method. However, this needs to be validated further in prospective series.
CLINICAL IMPLICATIONS
Detailed knowledge of the LAA geometry and accurate LAA ostium sizing are crucial for the safety and efficacy of percutaneous LAA occlusion. Definition of a good reference standard to size the LAA and decide the device size has not been established. By providing 3D images with high spatial resolution, MDCT enables more accurate measurements of the rather oval LAA ostium than 2D echocardiography. The frequency of periprocedural complications due to inadequate sizing and catheter manipulation may be minimised. Moreover, with MDCT the preprocedural suitability of the patient for both devices could be accurately assessed so that complicated procedures could be prevented in advance. Prospective studies randomising patients to LAA measurement with conventional 2D TEE or to 3D imaging techniques and evaluating the procedural results based on the imaging modality used may be of interest.
Limitations
The present evaluation comprised non-valvular AF patients who were referred for radiofrequency catheter ablation. Future, prospective studies are needed to determine the impact of preprocedural planning and size selection with MDCT on the safety and efficacy of percutaneous LAA occlusion. In addition, 2D TEE data were not systematically available. Furthermore, the present evaluation did not include a control group to compare the outcomes of transcatheter LAA closure when using TEE or MDCT to select the device size. In addition, mild residual leaks after transcatheter LAA closure have been reported in up to 32% and 16.2% of patients treated with the Watchman and Amplatzer Cardiac Plug devices, respectively22,23. Whether selection of a larger device size based on MDCT measurements would have resulted in no leakage remains unclear and needs to be investigated further in prospective series.
Conclusions
MDCT enables an accurate assessment of the LAA geometry and dimensions and may impact on procedural feasibility for both LAA occlusion devices.
Funding
The Department of Cardiology received research grants from Biotronik, Medtronic, Boston Scientific, BMS Medical Imaging, Edwards Lifesciences, St. Jude Medical & GE Healthcare.
Conflict of interest statement
V. Delgado received consulting fees from Medtronic and St. Jude Medical. The other authors have no conflicts of interest to declare.

