Abstract
Aims: Transoesophageal echocardiography (TEE) and multidetector computed tomography (MDCT) currently serve as imaging modalities for left atrial appendage (LAA) occlusion preprocedural planning. We assessed the feasibility of MDCT-based models to predict the correct size of device for LAA occlusion procedures.
Methods and results: Patients planned for LAA occlusion underwent MDCT before implantation, which was used for creating and printing 3D LAA models. Three cardiologists evaluated the 3D models and predicted the correct size of the device by manual manipulation. These predictions were compared with the actual device implanted during the procedure. Twenty-nine patients were included in this study. AMPLATZER and WATCHMAN devices were deployed in 12 and 17 patients, respectively. Two procedures were aborted due to failure of occlusion; all three physicians predicted it. There was good correlation between the 3D models and the inserted device for AMPLATZER devices with a concordance correlation coefficient of 0.778 (p=0.001) and poor agreement for WATCHMAN devices – concordance correlation coefficient of 0.315 (p=0.203). Agreement among the three physicians for AMPLATZER and WATCHMAN devices was excellent, with a calculated average intra-class correlation of 0.915 and 0.816, respectively.
Conclusions: We found LAA printed 3D models to be accurate for prediction of LAA occluder device size for the AMPLATZER device but not for the WATCHMAN device.
Abbreviations
AF: atrial fibrillation
ICC: intra-class correlation coefficient
LA: left atrium
LAA: left atrial appendage
MDCT: multidetector computed tomography
OAC: oral anticoagulants
STL: stereo lithography
TEE: transoesophageal echocardiography
VR: volume rendered
3D: three-dimensional
Introduction
Atrial fibrillation (AF), and thromboembolic stroke as its leading complication, causes significant mortality and morbidity, mainly due to emboli dislodged from the left atrial appendage (LAA)1. The LAA occlusion procedure has emerged as a safe and effective alternative to oral anticoagulants (OAC) in selected patients: recent experience has shown an up to 95% procedure success rate and >60% risk reduction in bleeding events2.
Anatomical studies have described the LAA as a blind-ended, tubular, trabeculated multiple-lobed structure, varying significantly between patients, making percutaneous occlusion procedures complex and challenging3. Transoesophageal echocardiography (TEE) and multidetector computed tomography (MDCT) currently serve as the imaging modalities for preprocedural planning, but predicting correct device size remains challenging4. Printed 3D models, based on anatomical data derived from MDCT scans, have previously been described mainly for structural heart disease imaging, but are not used routinely for LAA occlusion procedures5.
The purpose of the current study was to evaluate the feasibility of printed 3D LAA models to size the LAA occluder correctly for LAA occlusion procedures.
Methods
All patients included in this study were referred for an LAA occlusion procedure due to atrial fibrillation, and a contraindication to OAC. The choice of the implanted occluder type was based on availability and procurement issues at the time of the implantation. Device size was determined by a combination of intraprocedural TEE and LAA angiography. All patients underwent MDCT prior to implantation which was used for constructing the 3D models. Patients were not hydrated intravenously either before CT or before the occluder implantation. This study was approved by the institutional review board and included a dedicated informed consent.
MDCT 3D MODEL CREATION
MDCT was performed 10-14 days before the procedure, using a 256-slice scanner (Brilliance iCT; Philips Healthcare, Cleveland, OH, USA), and 80 ml of non-ionic contrast medium given intravenously. Systolic phases of the R-R interval (30-40% of the R-R interval) were utilised for analysis, and images were loaded to a dedicated cardiac imaging application (Comprehensive Cardiac Analysis, Extended Brilliance Workspace [version 4.5]; Philips Healthcare) to create model-based segmentation. Segmentation was edited and reconstructed, resulting in segmented models consisting solely of the wall of the aforementioned structures (Figure 1). This model was saved as a stereo lithography (STL) file and printed using a 3D printer (Objet, Rehovot, Israel), utilising a rubber-like material which has previously been used to construct cardiac 3D models (TangoPlus FLX930; Stratasys GmbH, Rheinmünster, Germany).
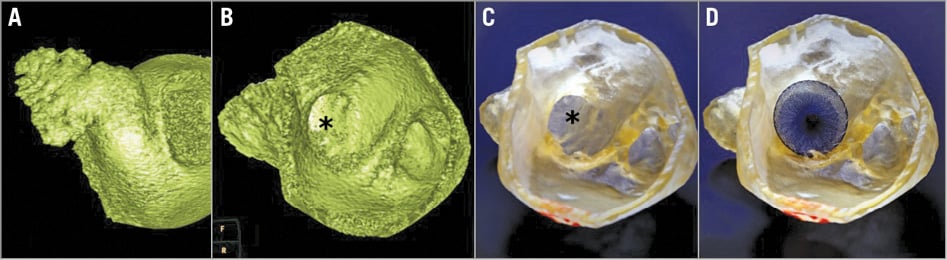
Figure 1. Example of a model of the LAA in a patient treated with the AMPLATZER device. A) LAA volume-rendered MDCT model. B) LAA volume-rendered interior MDCT model. C) LAA 3D STL model. D) LAA 3D STL model with AMPLATZER device inserted into the printed model. * LAA ostium
3D MODEL EVALUATION
Three separate experienced interventional cardiologists who routinely perform LAA occlusion procedures participated in this project. They were informed of the type of occluder used in the procedure (AMPLATZER™; St. Jude Medical, St. Paul, MN, USA, or WATCHMAN™; Boston Scientific, Marlborough, MA, USA), were blinded to the patient or procedural data, and were asked to use the printed 3D models in order to choose the appropriate occluder device size. Manual manipulation using the commercial delivery systems and the complete range of device sizes was used for estimation. The estimated device size was compared with the device size implanted during the procedure.
STATISTICAL ANALYSIS
The agreement between true device size and the average of the measurements of all three cardiologists and intra-observer agreement were estimated using Lin’s concordance coefficient and Bland-Altman limits of agreement for each type of device. Intra-observer agreement was also analysed by intra-class correlation coefficient (ICC). The data were presented as mean and 95% confidence interval for the mean. The concordance coefficient was considered as non-significant if the corresponding confidence interval included zero. All calculations were carried out using Stata/SE 12 software (StataCorp, College Station, TX, USA).
Results
The study cohort included 29 patients referred for LAA occluder insertion. The average age was 78±7 years, 64% were male. AMPLATZER and WATCHMAN devices were deployed in 12 and 17 patients, respectively.
Two procedures were aborted due to mismatch between LAA and any WATCHMAN device dimensions. All three physicians predicted these failures using the printed 3D model.
AMPLATZER MODELS (N=12)
There was a high correlation between the predicted device size using the STL model and the inserted device size. Lin’s concordance correlation coefficient was 0.778 (95% confidence interval [CI]: 0.384, 0.933; p=0.001). According to Bland-Altman analysis, the average difference between the predicted device size using the STL models and the inserted device was 0.848 mm (95% limit of agreement [LOA]: -4.215, 5.912) (Figure 2).
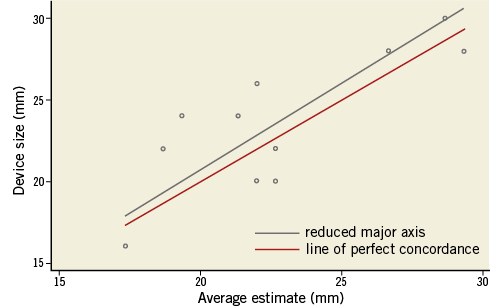
Figure 2. AMPLATZER device size estimation using LAA models Lin’s concordance coefficient expressing the compatibility between the average estimated device size (using the 3D STL LAA model) and the inserted device.
Agreement among the three physicians for the AMPLATZER device was excellent, with a calculated average intra-class correlation of 0.915 (95% CI: 0.559, 0.933).
WATCHMAN MODELS (N=17)
There was a lower correlation between the predicted device size using the STL model and the actual inserted device size. Lin’s concordance correlation coefficient was 0.315 (95% CI: -0.175, 0.680; p=0.203). According to Bland-Altman analysis, the average difference between the predicted device size using the STL models and the inserted device was 0.956 mm (95% LOA: –6.534, 8.445).
Agreement among all three physicians for the WATCHMAN device was good, with a calculated average intra-class correlation of 0.816 (95% CI: 0.559, 0.933).
Discussion
The LAA anatomy is complex, varying significantly between individuals. Therefore, it seems that 3D imaging modalities appear to be superior to 2D LAA imaging5. Currently available dedicated workstation tools allow visual demonstration of 3D models, which can be rotated and manipulated in relation to the viewing angle, but they lack the ability to feel and manipulate the device in a similar way to the actual procedure.
The current study sought to evaluate a novel approach using 3D STL printed models in order to predict optimal occluder device size. Recently, a letter to the editor describing a single patient addressed the potential of a 3D printed model for the prediction of the inserted occluder device6, but our study is the first case series assessing this concept.
We found the printed models to be highly accurate for AMPLATZER devices but not for WATCHMAN devices in predicting the actually inserted occluder size. However, strong agreement among physicians was demonstrated for both devices. The reason for the lack of accurate prediction of inserted device size for the WATCHMAN device is not clear from our data. Additionally, based on the printed 3D STL models, all three physicians predicted two procedural failures using WATCHMAN devices. This holds some promise that the models might help to avoid the discomfort, risks and cost of unnecessary procedures.
Limitations
This study has several limitations. The cohort is small and the models were printed using a semisolid synthetic material, with partial similarity to the human tissue (specifically the ability of the model to expand and adapt to the implanted device). The device implantation within the STL model utilised manual manipulation of devices, which is quite different from delivery systems and the implantation technique used in vivo. This might have affected device size selection. This study also lacks follow-up data which might shed additional light on device appropriateness.
Conclusions
This study demonstrated the potential role of printed 3D STL models for preprocedural planning, using existing 3D MDCT data sets. The printed models had high accuracy in predicting the size of AMPLATZER but not WATCHMAN devices, and can have a role in predicting procedural failure. Larger trials are needed to define the impact of preprocedural use of STL models in clinical practice.
| Impact on daily practice Determining the size of device for LAA occlusion procedures remains a challenge. We used MDCT-based printed 3D models and manual manipulation to predict the size of device that would be implanted during the procedure. We found the models to be accurate for prediction of device size for the AMPLATZER device but not for the WATCHMAN device. They were also accurate in predicting procedural failures. This study demonstrated the potential role of printed 3D models in preprocedural planning of LAA occlusion procedures. Further studies are needed in order to establish the impact of these models. |
Acknowledgements
We wish to acknowledge the contribution of Vladimir Perhulov, MD, to the visuals.
Funding
This work was supported by a grant from Philips Medical Systems (grant number 0365-13-SMC).
Conflict of interest statement
The authors have no conflicts of interest to declare.

