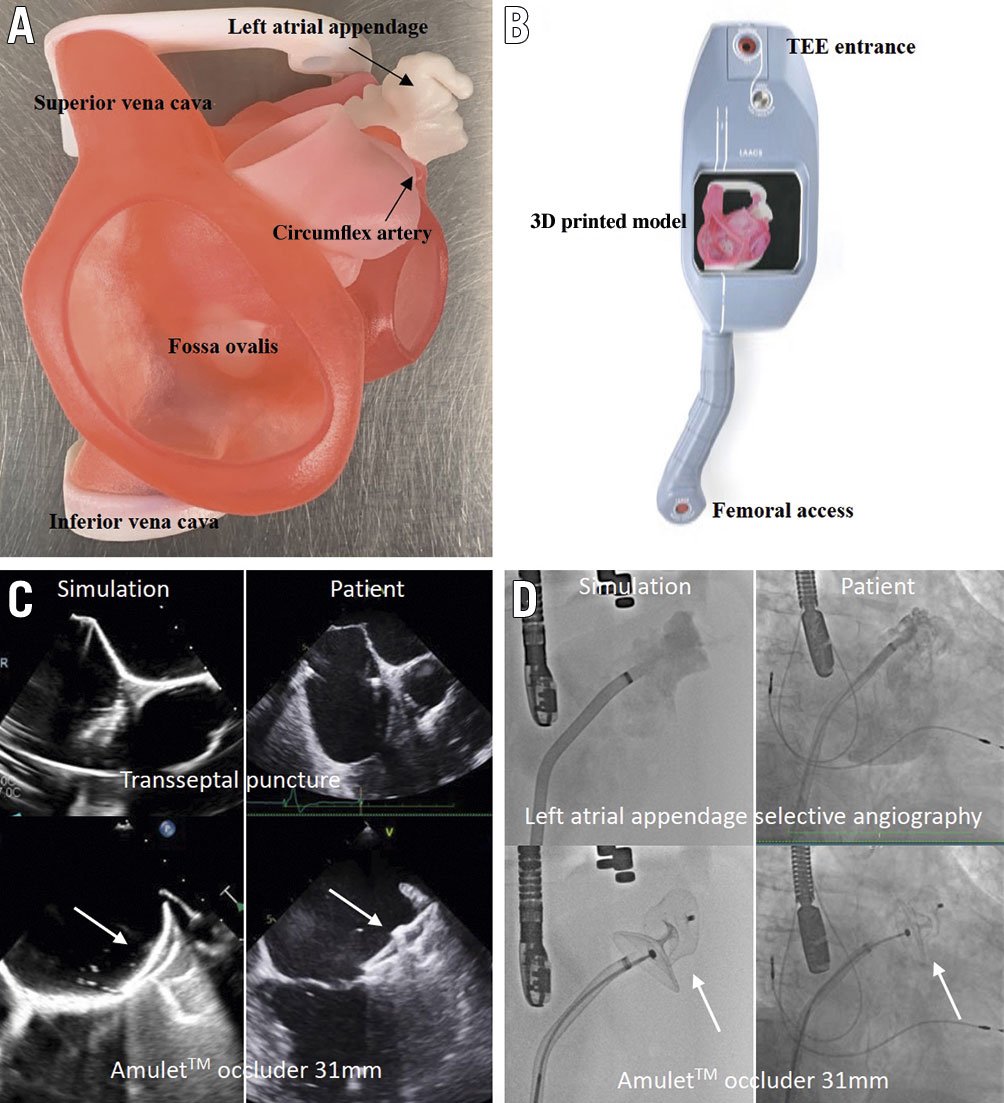
Figure 1. Simulation station with very realistic echo and fluoro imaging. A) 3D printed anatomy. B) BIOMODEX station. C) TEE views during transseptal puncture (45°) and of the Amulet occluder 31 mm on the BIOMODEX model versus the real patient. D) LAA selective angiography and fluoroscopic views of the Amulet occluder 31 mm on the BIOMODEX model versus the real patient.
Left atrial appendage occlusion (LAAO) procedural outcomes rely mainly on preprocedural LAA anatomy analysis and operator experience. Preprocedural multimodality imaging, including multislice computed tomography (MSCT), has become the current standard of care and impacts on procedural success1. Even though the effect of a learning curve has not been directly tested, the volume of LAAO procedures per centre has recently been inversely associated with major adverse events2. A need for skills training and preprocedural technical expertise development has emerged, particularly in structural heart interventions where case volume is still low3.
In this Interventional flashlight paper, we describe a successful case of percutaneous LAAO using a patient-specific 3D printed model with realistic imaging and haptic feedback in a 72-year-old man. All clinical, echocardiographic and computed tomographic (CT) data of patients treated for LAA occlusion in our institution are collected in a database approved by the institutional review board for research purposes. For this case, we also obtained patient approval for recording, diffusion and publication. The LAACS (Left Atrial Appendage Closure System) is a new instrument designed by BIOMODEX (Paris, France) for physician education, training, R&D and rehearsal. The system is compact, portable, intended to be used in a cath lab and is compatible with all imaging guidance technologies. The 3D model reproduces the anatomy from the MSCT images of the patient (Figure 1A). INVIVOTECH technology generates a multi-material distribution that recreates the deformation of the cardiac structures and ECHOTECH changes the texture and intensity of the material in order to mimic the acoustic properties of cardiac tissue. These optimisation algorithms are applied to the model to enhance the haptic (tactile) feedback experience and to obtain realistic echocardiographic imaging. The 3D printed anatomical twin includes the key transoesophageal echocardiography (TEE) landmarks needed to guide the procedure. Interestingly, the system is compatible with radiofrequency technology for transseptal puncture when salt is added to the water inside the tank.
The day before the real procedure, a simulation was performed successfully in the cath lab by the team eventually involved in the human case. All the steps of a real procedure were reproduced using the same equipment. More specifically, a VersaCross RF pigtail wire (Baylis Medical) was advanced through the right femoral vein (Figure 1B). Under TEE and fluoroscopic guidance, an inferoposterior transseptal puncture was performed. The D1 VersaCross sheath was then exchanged for an Amplatzer steerable delivery catheter (Abbott Vascular). Deflection allowed favourable coaxial alignment with the LAA ostium and a successful 31 mm Amulet (Abbott Vascular) device implantation. The simulation session successfully reproduced LAAO in a real-world setting with respect to similar LAA imaging, device type and size selection and final result (Figure 1C, Figure 1D). The use of the steerable sheath was mainly motivated by the simulation, which showed difficult co-axiality using the conventional sheath in this particular anatomy.
The BIOMODEX LAACS cartridge described in this case revealed biomechanics and fluid dynamics (4D) with acoustic properties (5D) that enabled the 3D printed anatomical twin to be clearly visible. Moreover, the operators reported impressive haptic feedback, leading to a safer and an easier human procedure by reducing the procedural time and by predicting the final result with no recapture and no missizing.
In the current era of rapid structural heart intervention development, simulation-based training will have a full part to play in the improvement of clinical and safety outcomes in the near future. Major limitations of currently available simulation platforms are the absence of patient-specific models and unrealistic tactile feedback. Patient-specific 3D printed models produced from MSCT or TEE imaging have been reported to improve prosthesis sizing accuracy as well as device implantation success45. However, technical complexity related to anatomical challenges may be underestimated by using preprocedural imaging analysis only. Simulation-based case planification using new platforms (BIOMODEX, Simulands) may adequately prevent complications associated with low-volume or inexperienced operators and complex anatomies, as well as optimising first-in-human procedures. The performance of the BIOMODEX platform to improve clinical and safety outcomes still needs to be assessed by specifically designed comparative studies of the LAAO procedure using, or not using, the simulation platform.
Acknowledgements
We would like to acknowledge BIOMODEX for the technical support.
Conflict of interest statement
R. Ibrahim is a proctor for Abbott and Boston Scientific and received research grants from Edwards Lifesciences and Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

