Introduction
Several strategies have been devised to obliterate the left atrial appendage (LAA) space, which is the suspected origin of the majority of cardioembolic thrombi in patients with non-valvular atrial fibrillation (NVAF)-related stroke1. The Omega™ LAA occluder (Vascular Innovations Co., Ltd., Nonthaburi, Thailand) is a novel self-expanding, cup-and-disc device made from a continuous, platinum-coated nitinol wire mesh (Figure 1), designed to occlude the LAA. The cup and disc are linked together by a flexible connecting waist. The disc has a polypropylene fabric securely sewn inside it, adding to the occlusive aspect of the device. For stabilisation, the cup carries 6 to 10 anchoring hooks. This study reports on the first-in-human experience and procedural safety and efficacy of the Omega LAA occluder.
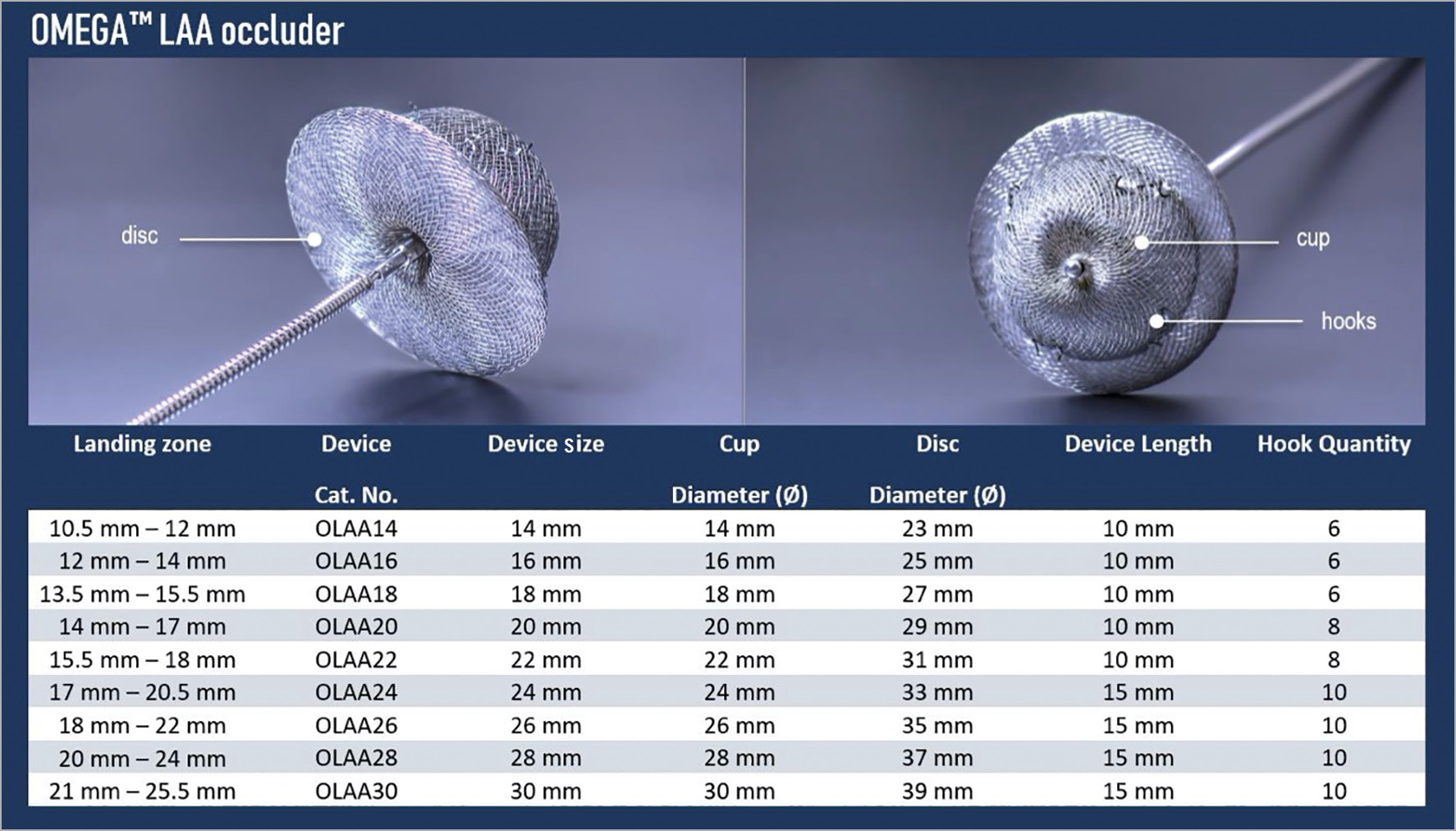
Figure 1. The Omega left atrial appendage occluder.
Methods
STUDY POPULATION
Patients were enrolled in an open-label, non-randomised trial at the King Chulalongkorn Memorial Hospital, Bangkok, Thailand, between February 2019 and October 2019. Eligible patients had confirmed NVAF with an indication for oral anticoagulation but were ineligible for this medical therapy due to a high bleeding risk. Exclusion criteria comprised both clinical and cardiac imaging features (Supplementary Table 1). The study protocol obtained approval from the local ethics committee and all patients provided informed consent.
PROCEDURE PLANNING
An ECG-gated, thin-sliced (≤1.0 mm) and contrast-enhanced cardiac computed tomography (CT) was performed in all patients and was analysed to measure the dimensions of the LAA orifice and LAA landing zone at a depth of 9 to 15 mm distal to the orifice – corresponding to the planned landing zone of the Omega anchoring cup. To enhance accuracy of device size selection further, a CT-based 3D silicone LAA model was created and used to simulate and test device deployment. A patient selection committee reviewed cases to ensure anatomical suitability.
DEVICE IMPLANTATION AND MEDICAL THERAPY
The Omega LAA occluder system consists of an occlusion device and a delivery system intended for transvenous femoral deployment with transseptal access to the left atrium (LA). Nine device sizes ranging from 14 to 30 mm (with 2 mm increments) are available and referred to by the maximal diameter of the cup, allowing anchoring in LAAs with a landing zone diameter between 10.5 and 25.5 mm. A minimum LAA depth of 10 mm to 15 mm is required for the Omega size 14 to 22 mm devices and size 24 to 30 mm devices, respectively (Figure 1). The delivery system consists of a 14 Fr double curve catheter that is introduced into the LA after transseptal puncture and when an activated clotting time of >250 seconds is reached. All patients received dual antiplatelet therapy for three months post procedure, followed by single antiplatelet therapy for a minimum of six months.
PRIMARY ENDPOINTS
The primary efficacy endpoint of the study was LAA closure (defined as either complete LAA seal or efficient seal with a peri-device leak <5 mm) at 30 to 90 days, as documented by transoesophageal echocardiography (TEE) with colour flow Doppler. The primary safety endpoint was device-related complications (adverse events) between 7 and 90 days post procedure. All study endpoints conform to the Munich consensus document on LAA closure research2.
Results
Among 18 patients screened, 13 patients were eligible to receive the Omega LAA occluder. Five patients were excluded due to one patient withdrawal, one LAA with insufficient depth, two LAA landing zones outside the intended size range, and one LAA with pre-existing thrombus. The baseline characteristics of the 13 enrolled patients are reported in Table 1.
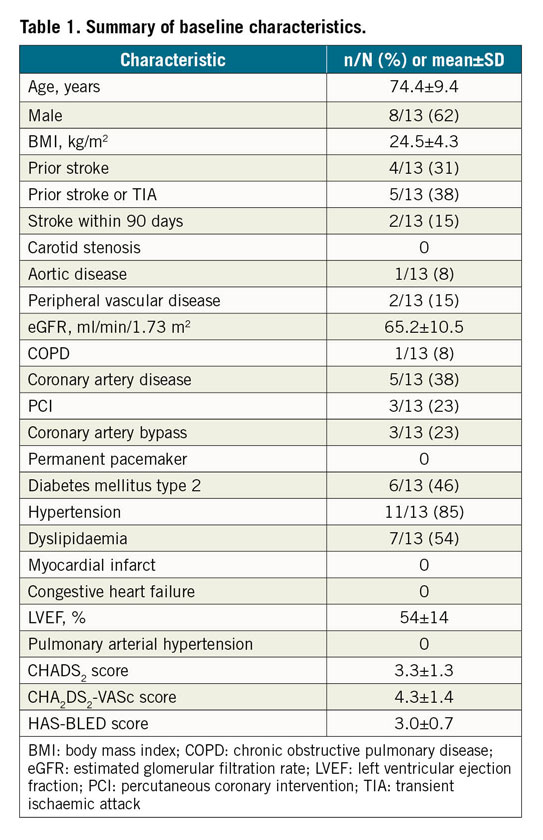
Percutaneous LAA closure was successful in all patients with a total mean procedure time of 59±16 minutes – including 21±8 minutes from introduction to removal of the delivery system, 6±3 minutes fluoroscopy time and the use of 101±31 ml of contrast dye (Table 2). Only one device per procedure was used in all procedures, requiring a mean of 1.7±0.9 deployment attempts before the final position was accepted. In seven patients (54%), a single initial deployment was accepted.
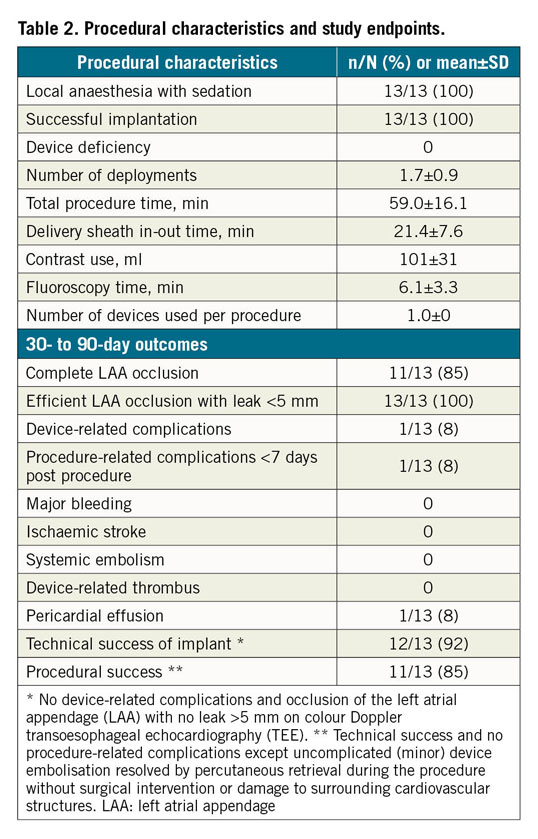
The primary efficacy endpoint of LAA closure at 30 to 90 days was obtained in all Omega recipients, with complete LAA closure in 11 patients (85%) and a small residual peri-device leak <3 mm in 2 patients (15%). The primary safety endpoint was noted in one patient who developed a periprocedural pericardial effusion, which was initially managed conservatively but drained percutaneously six weeks later. One patient had a local venous access haematoma, managed conservatively.
During follow-up, there was no device embolisation, major bleeding, device-related thrombosis, ischaemic stroke or systemic embolism. Thus, technical success was noted in 12/13 patients (92%) and procedural success in 11/13 patients (85%) (Table 2).
Discussion
First-in-human experience with the Omega LAA occluder shows a favourable safety and efficacy profile with successful device implantation, effective LAA closure and no device deficiencies in all cases. In the majority of procedures, the first device deployment was accepted.
The design features of the Omega LAA occluder provide a conformable device, targeting close wall apposition and effective LAA seal (Figure 2). A unique feature of the Omega device is the very flexible waist between the cup and disc, permitting use in a wide range of LAA anatomies, including sharply angulated LAAs/landing zones. This may provide an alternative to currently available LAA closure devices – such as the WATCHMAN™ (Boston Scientific, Marlborough, MA, USA), AMPLATZER™ Amulet™ (Abbott Vascular, Santa Clara, CA, USA) and LAmbre™ (Lifetech Scientific [Shenzhen] Co., Ltd., Shenzhen, China) – and provide effective LAA seal in a broader range of patients than currently treatable. Additional 32 mm and 34 mm Omega device sizes are planned.
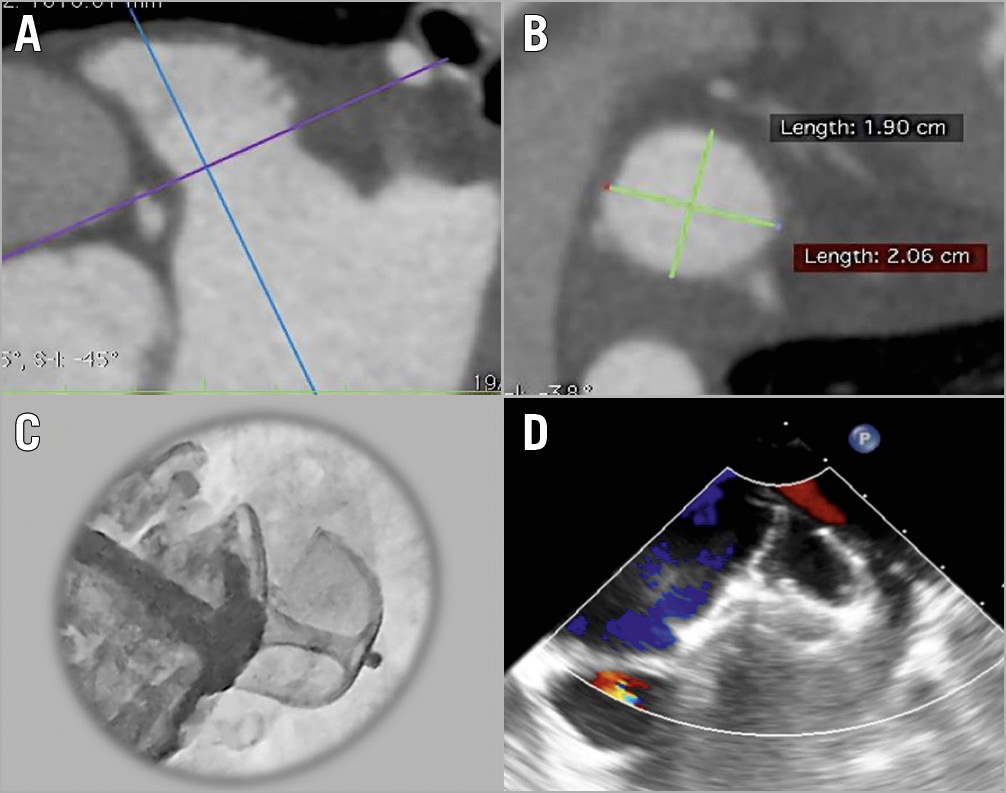
Figure 2. Procedural planning and implantation of the Omega left atrial appendage (LAA) occluder. A) Cardiac CT identification and assessment of LAA anatomy. B) Measurement of the LAA landing zone. C) In vivo deployment of the Omega device showing occlusion of the LAA with contrast injection. D) Procedural transoesophageal echocardiography showing the deployed Omega device and no residual flow into the LAA.
Limitations
This study is limited by the small number of patients and single-centre experience, as is often the case for first-in-human studies. The presence of a selection committee to select appropriate LAA anatomies to receive the Omega device may limit the generalisability of these study outcomes. Larger studies investigating long-term clinical efficacy and ongoing safety monitoring are planned.
Conclusion
Initial human experience with the Omega LAA occluder is favourable with all patients meeting the pre-specified primary efficacy endpoint of LAA closure immediately post procedure and at 30- to 90-day echocardiographic evaluation.
|
Impact on daily practice As experience with LAA closure increases, it is hoped that additional technologies, including the Omega LAA occluder, will increase procedural safety and improve patient outcomes. |
Acknowledgements
Ben Wilkins is supported by a training and research grant from the Heart Foundation of New Zealand [grant # 1778].
Funding
The study was funded by Eclipse Medical, Ireland, and Vascular Innovations Co. Ltd, Thailand.
Conflict of interest statement
L. Søndergaard is a shareholder in Eclipse Medical. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

