Introduction
In this chapter of Tools & Techniques Clinical, transcatheter closure of left atrial appendage using the Amplatzer™ Cardiac Plug is discussed using a stepwise approach. The following is a summarised overview of this technique. The complete, unabridged version with images is available online at www.eurointervention.org
Background
Since chronic oral anticoagulation cannot be administered in a substantial proportion of patients with atrial fibrillation, the endovascular exclusion of the left atrial appendage (LAA), which represents the source of atrial-derived emboli in the majority of cases, has been proposed as a valuable alternative in selected patients. In this article we briefly summarise the basic steps to perform an effective and safe implantation procedure using the Amplatzer™ Cardiac Plug (ACP; St. Jude Medical, Plymouth, MN, USA).
Methods
First, both cardiac computed tomography and transoesophageal echocardiography should be complementarily performed to study LAA anatomy and size accurately (Figure 1). LAA dimensions must be validated and confirmed during the procedure either by TEE or intracardiac echo (ICE) and angiographic measurements. It is recommended that the Amplatzer™ Cardiac Plug be oversized by about 2 to 3 mm with respect to the LAA measurements. Second, a transseptal puncture, using either the Mullins sheath (Medtronic AVE, Galway, Ireland) or the SL1 sheath (St. Jude Medical, Plymouth, MN, USA), should be performed in the lower part of the fossa ovalis. Third, after the advancement of a marker pigtail catheter (5 Fr, 100 cm long) through the transseptal sheath and the localisation of the pigtail catheter inside the LAA, perform an LAA angiogram to size the LAA ostium and “neck” angiographically. Fourth, prepare the LAA device accurately in a step-by-step fashion (as described in the on-line extended version of the paper) (Figure 2). Fifth, advance the delivery sheath using an Amplatz Super Stiff™, 0.035”×260 cm, short J tip guidewire (Boston Scientific, Natick, MA, USA). Sixth, identify the “landing zone”, i.e., the place where the ACP lobe will be implanted, and carefully unsheath the device until it forms a “ball”. With the device in the “ball” position you can safely manipulate the system in order to prepare for the ACP lobe delivery. Seventh, if the ACP lobe position is satisfactory, unsheath the ACP disc applying some traction to the delivery wire. The goal is to have the lobe of the device completely inside the LAA, with a slight deformation of its body-tyre-shape and with its major axis perpendicular to the major axis of the landing zone. Contrast media injection through the delivery sheath can confirm the correct position of the device and the effectiveness of LAA occlusion. If first attempts are not successful, the device can be safely repositioned at any stage until it is not retracted farther than the radiopaque markers without changing the device or delivery system. Eighth, it is important to verify the device stability before its release, thus keeping the proximal disc on traction for at least three minutes. Ninth, after echocardiographic and fluoroscopic confirmation that the device is correctly in place, it can be released by turning the delivery cable vice counterclockwise (Figure 3). Tenth, a check for pericardial effusion at the end of the procedure is done by transthoracic echo (TTE). We refer to the on-line version of the manuscript for the management of possible periprocedural complications as well as for patient discharge and follow-up. In our experience with the Amplatzer™ Cardiac Plug, implantation is safe and uneventful if performed by a multidisciplinary team and according to a standardised procedure as described in this paper.
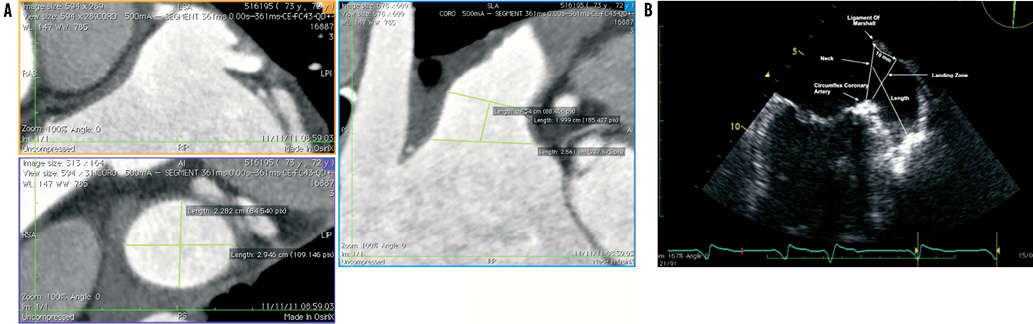
Figure 1. A) Transversal projection and 3-D multiplanar measurements; B) Transoesophageal echocardiography measurements of left atrial appendage.
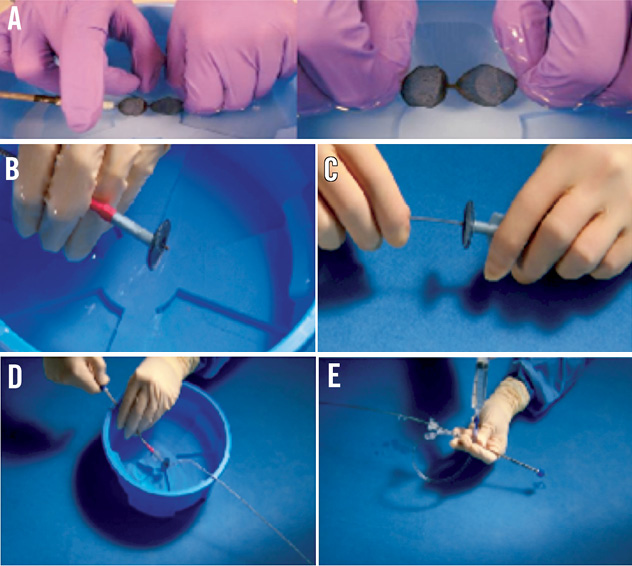
Figure 2. A) Manipulation of the Amplatzer Cardiac Plug device in sterile saline solution by hand to eliminate air bubbles; B) Amplatzer Cardiac Plug – lobe fully retracted within the loader; C) Delivery cable connected to the exposed proximal end screw of the device; D) Pulling of the loading cable to recapture the disc into the loader; E) Flushing of the loader assembly through the haemostatic valve.
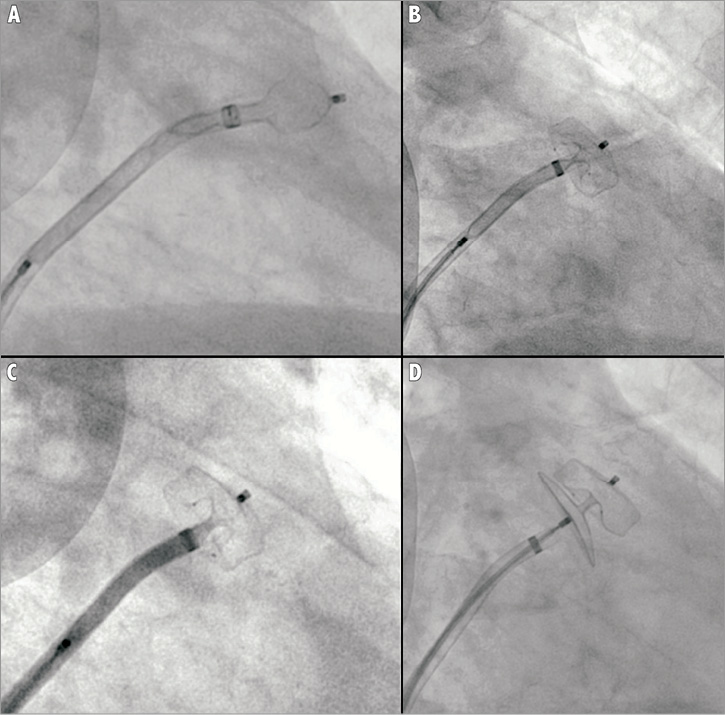
Figure 3. A) Angiography of Amplatzer Cardiac Plug – lobe “ball” shape; B) Deployment of Amplatzer Cardiac Plug – lobe; C) Angiography of Amplatzer Cardiac Plug – lobe positioned RAO 40° – caudal 20°; D) Deployment of Amplatzer Cardiac Plug – disc.
Conflict of interest statement
The authors report no potential conflicts of interest with respect to any companies/organisations whose products or services have been discussed in this article.

