The prevention of stroke is a primary goal in patients with atrial fibrillation (AF) because of its high morbidity and mortality. Cardiac embolism due to AF causes up to 25% of all ischaemic strokes underlining its supplement socio-economical relevance1. The prevalence of AF increases due to the rapidly growing proportion of elderly people. Apart from heart rate control and anticoagulation, transcatheter left atrial appendage (LAA) closure is a newly accepted therapeutic option in selected patients with permanent or paroxysmal AF who have contraindications for anticoagulation therapy and a high cardioembolic risk2,3.
Valvular heart disease, especially aortic valve stenosis (AVS), is also frequently seen in elderly patients. Lately, new interventional techniques like percutaneous transcatheter aortic valve implantation (TAVI) offer alternative treatment strategies to patients with AVS, which might avoid major surgery and long-time anticoagulation therapy in patients with relevant comorbidities.
The catheter interventional management of both entities emerges as a possible strategy to maintain quality of life and to reduce hospitalisation in elderly patients4.
We present the case of an 82-year-old male patient with a history of chronic AF since 1999. During anticoagulation therapy with warfarin he developed a subdural haematoma in 2002. He recovered completely and was on aspirin as the only antithrombotic treatment since that episode. In 2005, a relevant AVS was detected leading to cardiac decompensation in early 2010. Consequently, transfemoral TAVI was performed in March 2010 using a 29 mm CoreValve® (Medtronic, Minneapolis, MN, USA) prosthesis. Post-procedural echocardiography showed a normal systolic and diastolic function.
One day after TAVI a pacemaker had to be implanted because of bradyarrhythmia. In view of the history of cerebral bleeding and the 6-month clopidogrel therapy following TAVI, we decided not to extend anticoagulation therapy by warfarin. Two months later, percutaneous closure of the LAA with the 24 mm Amplatzer Cardiac Plug® (ACP) (AGA Medical Corporation, Plymouth, MN, USA) was done uneventfully (Figure 1 and Figure 2).
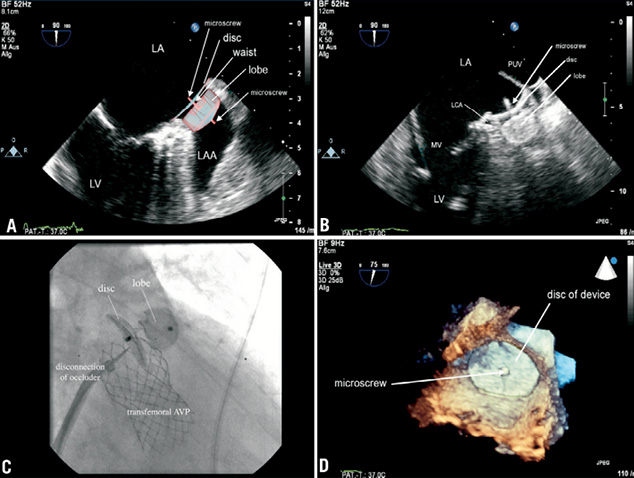
Figure 1. 2D biplane transesophageal images (A) showed the transseptal puncture site of choice to reach LAA directly. To find the optimal occluder size, the entrance (B) and depth of the LAA were measured. 3D real-time TEE (C) provided supplement information for a further improved guidance and positioning of the occluder. An angiogram of the LAA (D) completed the necessary information. LV: left ventricle; MV:mitral valve; AVP: aortic valve prosthesis; LA / RA: left atrium / right atrium; LAA: left atrial appendage; IAS: inter-atrial septum; LCA: left coronary artery; 3D-RT-TEE: three-dimensional real-time transesophageal echocardiogram; PUV: pulmonary vein; CDE: colour Doppler echocardiogram; ACP: Amplatzer Cardiac Plug
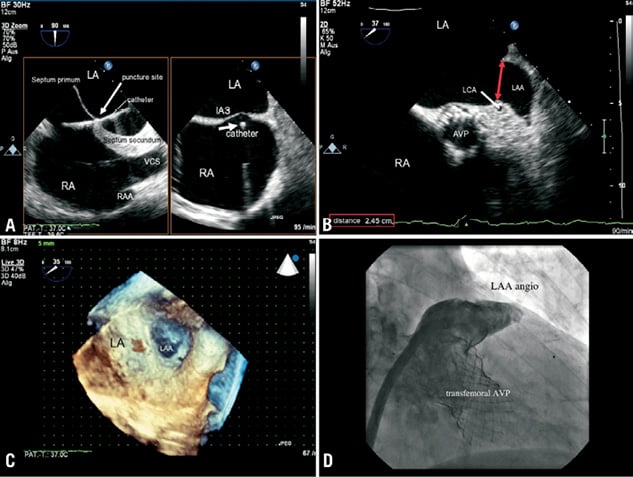
Figure 2. The ideal positioning of the occluder is a complete coverage of the LAA entrance (A). In our case, the implanted 24 mm ACP covered nearly the whole entrance of LAA (B) apart from the area of the thin wall between LAA and left pulmonary vein. The lobe of the device was clamped within the LAA demanding a minimal positioning depth of 10 mm. Therefore, in our case the device was implanted slightly deeper within the LAA to fix the lobe definitely. Compression of the left main coronary artery (LCA) is a possible complication. Before releasing the ACP (C) x-ray confirmed the correct position by a dragging manoeuvre (Moving image 1). TAVI did not interfere with the ACP. 3D-real-time TEE (D) finally demonstrated the proper position of the device (Moving image 2). Proof of device competence and exploration for an iatrogenic ASD should follow routinely (Moving image 2). LV: left ventricle; MV:mitral valve; AVP: aortic valve prosthesis; LA / RA: left atrium / right atrium; LAA: left atrial appendage; IAS: inter-atrial septum; LCA: left coronary artery; 3D-RT-TEE: three-dimensional real-time transesophageal echocardiogram; PUV: pulmonary vein; CDE: colour Doppler echocardiogram; ACP: Amplatzer Cardiac Plug
Closure of LAA by ACP is an alternative treatment option in patients with AF, even in cases with a history of TAVI, as it offers the opportunity to avoid warfarin therapy, reduces the incidence of cerebral events and decreases the rate of hospitalisation. This can contribute to an improved cost-effective strategy despite the initially high consumption of resources.
Conflict of interest statement
The authors have no conflict of interest to declare.
Online data supplement
Moving image 1. The moving image shows the investigation and closure of the LAA by multimodal synchronous imaging, first demonstrating the angiogram and the rotating 3D-real-time TEE of LAA. In the 2D TEE, the implantation of the ACP-device into the LAA with the final opening of the disc of the device is shown. To ensure a proper fixation of the occluder’s lobe, the fixation proof manoeuvre was demonstrated by 2D TEE, 3D-RT-TEE and finally x-ray, which were monitored synchronously.
Moving image 2. The moving image shows the final situation after implantation of the ACP device. Conventional 2D TEE and 3D-RT-TEE, show the implanted device with the application catheter still connected to the microscrew of the device. No compression of the LCA was detected, although the discus of the ACP device is next to this structure. Residual flow was then searched for within the LAA using CDE and an angiogram was performed which demonstrated a very little amount of contrast flowing through the nitinol-net of the ACP-discus. Finally, the control of the IAS puncture site by CDE revealed a neglectable iatrogenic left to right shunt.

