Abstract
Percutaneous left atrial appendage (LAA) closure is becoming a frequently performed procedure for patients with atrial fibrillation and high haemorrhagic risk. The Amplatzer™ Cardiac Plug (ACP) is one of the most commonly used devices for this purpose. Despite high success rate and low procedure risk associated with the ACP, a second generation of the device is now available. The new ACP has been designed to facilitate the implantation process, improve sealing performance and further reduce the risk of complications. The present report focuses on the novel features of the second generation of the Amplatzer™ Cardiac Plug (ACP 2 or Amulet™) and describes the first-in-man experience.
Introduction
Percutaneous left atrial appendage (LAA) closure is considered an alternative to anticoagulation in patients with atrial fibrillation at risk of stroke or peripheral embolisation1. Although LAA closure is a relatively new technique, its usage is rapidly expanding worldwide2-5.Currently, the Amplatzer™ Cardiac Plug (AGA, St. Jude Medical, Minneapolis, MN, USA) and the Watchman™ (Atritech, Boston Scientific, Natick, MA, USA) are the two devices with most published data. The first generation of the Amplatzer™ Cardiac Plug (ACP 1) presented a particular design with a distal lobe and a proximal disc conceived for sealing the body and ostium of the LAA, respectively (pacifier effect). This design was based on the initial use of double disc Amplatzer devices for LAA closure6. Although reported periprocedural complication rates are low with the ACP 12-4, major adverse events such as procedural stroke, device embolisation, pericardial effusion and device thrombosis are still noted2,7. In addition, the highly variable anatomy of the LAA represents a challenge for device sizing and implantation. The new generation of the Amplatzer™ Cardiac Plug, Amulet™ (ACP 2) has been designed with strategic modifications to facilitate the implantation process and minimise the occurrence of complications without changing the main design of the ACP 1. The present report describes the novel features of the ACP 2 device as well as the first-in-man experience.
AMPLATZER™ CARDIAC PLUG 2: DEVICE DESIGN AND NOVEL FEATURES
The ACP 2 is a self-expanding device specifically designed for LAA closure. The main design, made of a nitinol mesh with two polyester patches sewn on to a distal lobe and a proximal disc connected by a short waist, has been carried over from the first generation device. Similarly to the first generation, the ACP 2 is implanted through the femoral vein via the transseptal technique, and is fully retrievable and repositionable. The modifications leading to the ACP 2 design, as shown in Table 1 and Table 2, are the following: 1) no need to prepare and load the device as it comes pre-loaded inside the delivery system; 2) the length of the distal lobe is 2 to 3 mm longer than the ACP 1 (Figure 1); 3) the stabilising wires (hooks) are stiffer; 4) the number of stabilising wires has been increased from six pairs in the ACP 1 to up to 10 pairs; 5) the diameter of the proximal disc has been increased in the ACP 2, now being 6 to 7 mm greater than the distal lobe diameter compared to 4 to 6 mm in the ACP 1; 6) the waist between the distal lobe and the proximal disc has also been lengthened from 4 mm in the ACP 1 to 5.5 mm or 8 mm depending on the size of the device (Figure 1); 7) the attaching screw on the proximal disc has been inverted (Figure 1); 8) the ACP 2 has a new delivery cable with an inner 0.014” wire8 (Figure 2); and 9) larger sizes are available (31 mm and 34 mm). These modifications to the design of the original device were made to facilitate implantation and improve sealing performance.

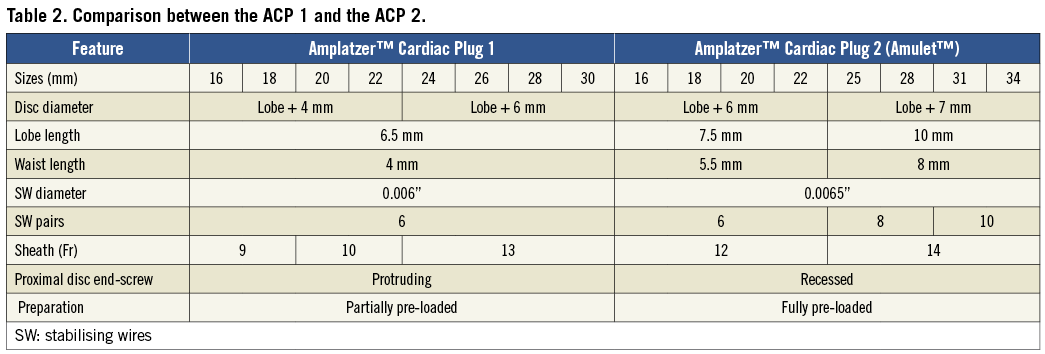
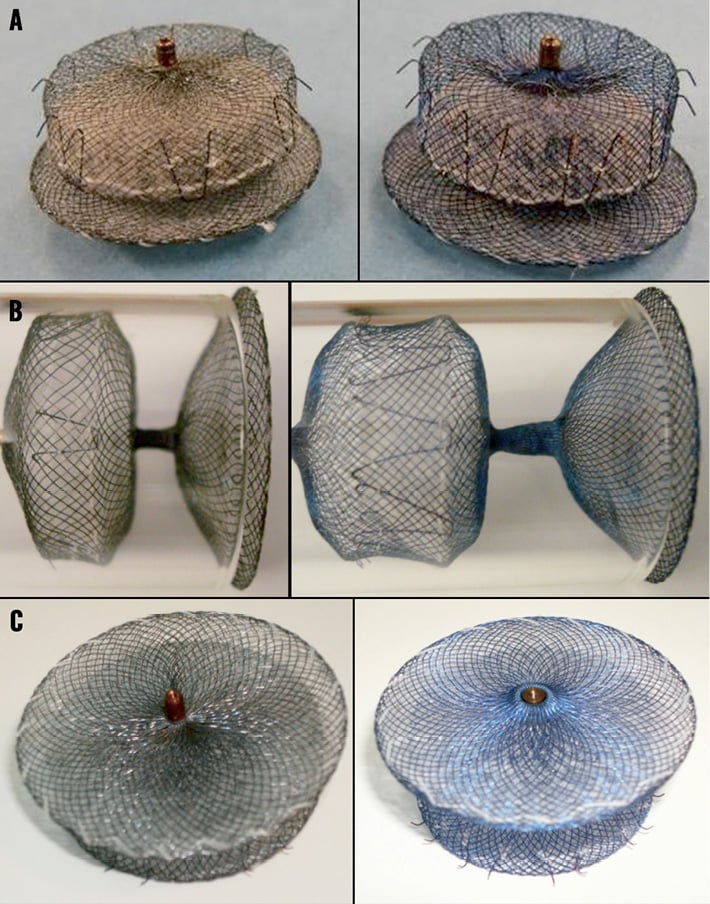
Figure 1. Comparison between the ACP 1 and the ACP 2. Comparison between the ACP 1 (left) and the ACP 2 (right) highlighting the greater diameter of the ACP 2 distal lobe (A and B) and waist (B), the increased number of stabilising wires (A) and the inversion of the disc end-screw (C).
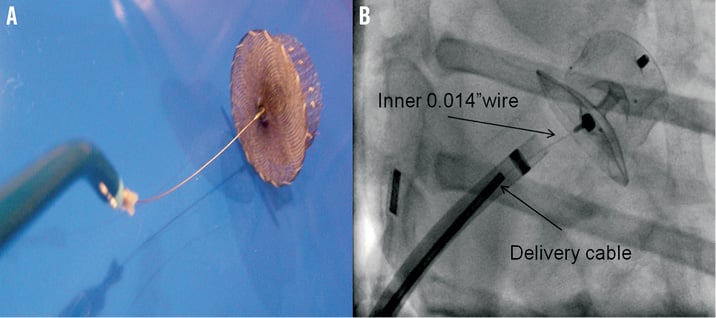
Figure 2. ACP 2 delivery system. ACP 2 delivery system with an inner 0.014” wire within the delivery cable that allows optimal assessment of final device position after releasing the tension from the delivery system.
CASE REPORT
The first-in-human percutaneous LAA closure using the ACP 2 was performed at the Montreal Heart Institute on July 19th 2012. The first patient was a 67-year-old woman, who had a history of chronic atrial fibrillation, hypertension, diabetes and aortic valve replacement with a biologic valve in 2011. She had no prior history but was at high risk of stroke or peripheral embolisation (CHADS2=3 and CHA2DS2VASc=5). Oral anticoagulation was substituted by aspirin and formally contraindicated after massive gastrointestinal bleeding secondary to angiodysplasia. Because of high stroke risk without oral anticoagulation, LAA device closure was considered to be indicated.
Transoesophageal echocardiography (TEE) was performed two days before the procedure to rule out the presence of LAA thrombus and to assess the dimensions and the morphology of the LAA. Device closure was performed under general anaesthesia and TEE guidance. As shown in Figure 3, LAA dimensions of 22.1 mm at the ostium and 20.0 mm at a depth of 10 mm from the ostium (i.e., landing site of the device lobe) were measured by TEE.
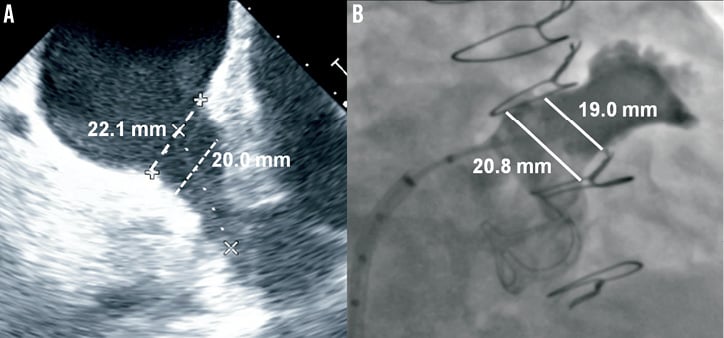
Figure 3. Left atrial appendage size. Left atrial appendage diameters measured at the ostium and at a depth of 10 mm from the ostium by transoesophageal echocardiography at 120° (A) and angiography at RAO 30° Cranial 20° (B).
After cannulation of the right femoral vein, a low and posterior transseptal puncture was performed using an 8.5 Fr SL-1 sheath (St. Jude Medical) and a Brockenbrough transseptal needle (BRK™; St. Jude Medical). Heparin was then given to keep an ACT above 250 sec and the LAA was engaged with a marker 5 Fr pigtail catheter (Merit Medical, UT, USA) to perform selective angiograms (Figure 3). LAA dimension of 20.8 mm at the ostium and 19.0 mm at a depth of 10 mm were measured by angiography. A pre-loaded 22 mm ACP 2 device was chosen based on LAA morphology, angiographic and TEE measurements, and operator experience. After careful flushing of the loader, the ACP 2 device was introduced in a 12 Fr TorqVue™ 45°-45° delivery sheath (St. Jude Medical) and placed into the LAA. The device was then deployed and repositioned until an optimal position was achieved. Repositioning was felt to be simpler compared to that with the ACP 1, as the device provided more stability in every tested position. After confirming the absence of residual leak by angiography and TEE, the tip of the delivery cable was pulled back in the left atrium leaving only a floppy inner 0.014” wire attached to the device (Figure 4). This security feature allowed a second assessment of the projected final position of the device after removal of the tension in the system. The device was then completely released in the usual manner by turning the screw on the inner wire counter-clockwise. Final TEE and angiographic evaluations confirmed optimal ACP 2 positioning with absence of residual leak and without associated complications (Figure 4). Follow-up echocardiography at twenty-four hours demonstrated adequate device positioning without pericardial effusion. The patient was discharged the day after the admission with long-term therapy with a daily low dose (80 mg) of coated aspirin, and 75 mg a day clopidogrel for three months. Endocarditis antibiotic prophylaxis was also recommended for at least six months.
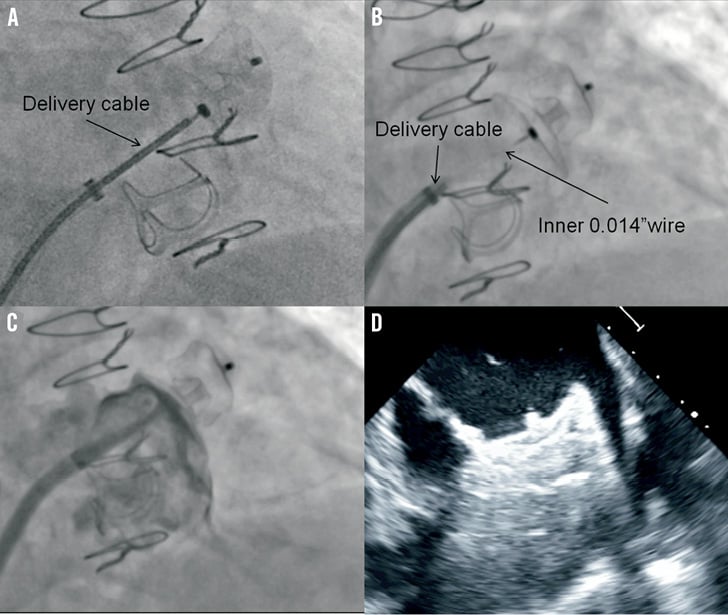
Figure 4. ACP 2 implantation. Deployment sequence of the ACP 2 implantation showing the initial position of the delivery cable (A), the inner 0.014” wire attached to the system after pulling back the delivery cable (B) and the final result after releasing the device without angiographic leak (C) and optimal echocardiographic positioning (D).
Discussion
The ACP 1 is a commonly used device in patients with atrial fibrillation and a formal contraindication to anticoagulation2-4,9. Despite very good results observed with the first-generation ACP, the call has been made by experienced operators to improve the device in order to reduce further the risk of periprocedural stroke (0 to 2.3%)2-4, device embolisation (0 to 2.3%)2-4, device thrombosis (0 to 2.4%)7,10 and pericardial effusion (1.1 to 3.5%)2-4. The ACP 2 (Amulet™) constitutes the second generation of the ACP and it has been redesigned based on three main principles: 1) to keep the existing ACP 1 platform but improving its performance; 2) to simplify the deployment process; and 3) to reduce the number of complications.
The ACP 2 design is similar to that of the previous generation consisting of a distal lobe which anchors inside the LAA and a proximal disc which seals the ostium of the LAA.
It has been noted that the procedural learning curve plays an important role in implantation success rate and the occurrence of complications1,2,11. An important novel feature of the ACP 2 is that it comes pre-loaded in the package, considerably facilitating and speeding up the set-up process and reducing the potential risk of air entrance. Indeed, the ACP 1 requires a complex loading process that demands a very close initial mentoring with a relevant learning curve. Device thrombosis appears to be most commonly associated with the protrusion of the metallic disc end-screw inside the left appendage7,10. In order to minimise this risk, the metallic screw is now “buried” inside the disc of the ACP 2 by inverting the position of the pin.
Most cases of embolisation of the ACP 1 have been linked to an inadequate implantation depth with partial protrusion of the lobe within the left atrium on the left circumflex artery side4,10. The presence of almost twice the number of stabilising wires (up to 10 pairs of hooks in the largest device), combined with a larger ACP 2 lobe diameter, is expected to provide greater device stability. Moreover, the distance between the lobe and the proximal disc has been increased, reducing the tension between the two components, giving more forgiveness and preventing multiple potential repositioning manoeuvres related to “pop-out” during the implantation process.
The relatively high incidence of post-implantation pericardial effusion, in up to 4.1% of cases, has been a matter of concern not only with the Watchman™ device1,11 but also with the ACP 12. A relevant learning-curve effect has been suggested as the incidence of pericardial effusion is reduced with increasing operator experience10,11. Simplification of device implantation with the ACP 2 should lead to a reduction of the number of manipulations inside the LAA and to a potential reduction of the risk of pericardial effusion. Nonetheless, the presence of more and stiffer hooks should always be taken into account as aggressive manipulations may result in a potentially higher risk of LAA damage.
Although data on optimal device sizing are still scarce, the general recommendation for the ACP 1 was to oversize from 1.5 to 3 mm in relation to the largest angiographic and TEE measurements. With the new ACP 2, the company recommends a larger oversize: 3 to 5 mm for 16 to 22 mm devices and 3 to 6 mm for 25 to 34 mm devices. This recommendation could lead to improved sealing and may also reduce the risk of embolisation related to undersizing. It is also important to point out the need for special care when selecting the size of the device in small and short LAA as a result of the longer length of the device (between 2.5 and 7.5 mm longer than the ACP 1).
Other interesting improvements with the ACP 2 include the addition of two larger devices (31 and 34 mm) (Table 2) and the presence of a brand-new delivery cable with a floppy inner distal 0.014” wire (Figure 2). The inner wire allows re-evaluation of the device orientation as most of the tension is released after pulling back the delivery cable while the occluder is still attached to the inner wire. If any concerns remain about the position, the delivery catheter can be re-advanced and the device can be repositioned or retrieved as needed. If, however, the final result is adequate, the device can be fully released by unscrewing the delivery wire with a counter-clockwise rotation.
The procedure was performed by an experienced operator with more than 30 ACP 1 implants. A 22 mm device was chosen, 3 and 2 mm larger than angiographic (19 mm) and TEE (20 mm) measurements. The final result was excellent without significant residual leak or pericardial effusion. The patient was discharged without any complication. Although the implantation was found to be improved in terms of simplicity and safety, no further subjective remarks are provided as this was a single case experience.
In summary, this manuscript focuses on the novel features of the ACP 2 and describes the first-in-man experience. Although we observed a great result, the performance and safety of the ACP 2 will need to be confirmed in larger series of patients.
Conflict of interest statement
R. Ibrahim is a consultant for Medtronic and a proctor for St. Jude Medical and Gore. A. Tzikas is a proctor for St. Jude Medical. The other authors have no conflicts of interest to declare.

