Abstract
Aims: Pericardial effusion (PE) without obvious periprocedural complications (e.g., cardiac perforation, device erosion) may occur after transcatheter closure of secundum atrial septal defects (ASD). The aim of the study was to investigate the incidence and predictors of PE unrelated to procedural complications.
Methods and results: We included all patients who had undergone successful percutaneous ASD closure from June 2009 to April 2014 (n=2,652) with no pre-existing PE or cardiac perforation or erosion. Transthoracic echocardiography (TTE) was performed during the procedure and one, three, and six months postoperatively. After device implantation, fifty patients (1.9%) developed new-onset PE (37 immediately, 13 during follow-up). These patients were asymptomatic, stable haemodynamically, and had no new arrhythmias. PE appeared mild (5.1±1.9 mm) and homogeneously echolucent by TTE. PE diminished spontaneously. Compared with 2,602 patients without PE, factors independently predicting asymptomatic PE were the device touching the atrial free wall, device size, patient age, and total defect size. Areas under the receiver operating characteristic curves were 0.78 (p<0.001), 0.66 (p<0.001) and 0.77 (p<0.001) for device size, patient age, and total defect size, respectively.
Conclusions: This is the first systematic report of a new type of PE. Our data provide new insights into new-onset PE after percutaneous ASD closure.
Abbreviations
ASD: atrial septal defect
AUC: area under curve
CI: confidence interval
PE: pericardial effusion
ROC: receiver operating characteristic
TEE: transoesophageal echocardiography
TTE: transthoracic echocardiography
Introduction
Pericardial effusion (PE) is a rare complication of transcatheter closure of a secundum atrial septal defect (ASD), with a reported incidence of 0.5-1.5%1-3. The reported aetiology ranges from wire perforation to catastrophic device erosion4. However, PE related to transcatheter closure of ASD without an obvious cause has only been described in case reports, and has not been the subject of systematic study1,5,6. The incidence and predictors of such PE unrelated to cardiac perforation or erosion are unknown. We sought to investigate the incidence and predictors of PE following percutaneous ASD closure. This study aimed to provide interventional cardiologists with a novel way to approach the diagnosis and differential diagnosis of new-onset PE after ASD closure.
Methods
PATIENT POPULATION
This is a retrospective study of 2,692 ASD patients who underwent transcatheter closure of secundum ASD at Fuwai Hospital, Beijing, China between June 2009 and April 2014. Eligible patients included those who had a haemodynamically significant secundum ASD. Patients with successful implantation of a device without significant complications during the procedure or follow-up were included. The significant complications were defined as device malposition, cardiac perforation or tamponade, device erosion, embolisation, and moderate to severe residual shunt. Patients who had other cardiac lesions or structural abnormalities as well as those in whom ASD closure was combined with other interventional operations were excluded7-9.
A total of 2,692 patients underwent percutaneous ASD closure (we excluded 40 patients who had pre-existing PE from this study). The remaining patients (n=2,652) –including 50 patients with new-onset PE that appeared during the procedure or the follow-up (PE+ group) and 2,602 patients without PE during the procedure or the follow-up (PE- group)– were studied retrospectively. Patients with procedural or follow-up PE (PE+ group) were reviewed in detail, including their clinical and echocardiographic features and their outcomes. They were compared with those free from PE (PE- group).
The local institutional review board approved the study. Written informed consent was obtained from all subjects.
PROCEDURE
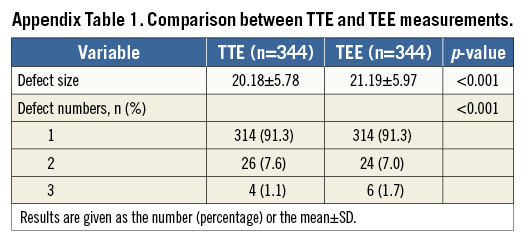
The fluoroscopy-guided implant procedure was carried out under local anaesthesia. Transthoracic echocardiography (TTE) was used for assessment and guidance of ASD closure. The entire procedure was assessed by TTE instead of using the “stop flow” balloon or transoesophageal echocardiography (TEE) sizing algorithm. Defect size and number, location of the device, and the presence of PE were determined by TTE. A series of TTE scans was performed before, during, and one day after device implantation. TEE was used for diagnosis only when the patient had a suboptimal acoustic window (Appendix Table 1). The interventional procedure was comparable to the international protocol except for the TTE algorithm10,11.
QUANTITATIVE ASSESSMENT OF THE DEFECT AND DEVICE
Criteria for the device size were as follows: for adults, device size=defect size +6-8 mm, and for children (<18 years) device size=defect size +3-4 mm12,13. The maximum defect size was used, as measured by TTE. When multiple defects were present, the size of each defect was calculated and added together and the total defect size was used.
Four devices were used, namely the AMPLATZER® Septal Occluder (AGA Medical Corporation, Plymouth, MN, USA), Cardi-O-Fix Septal Occluder (Starway Medical Technology, Beijing, China), HeartR™ Septal Occluder (Lifetech, Shenzhen, China), and Shanghai Shape Memory Alloy Septal Occluder (Shanghai Shape Memory Alloy Co., Ltd., Shanghai, China). The last three devices were manufactured in China and are described in the Results and Discussion sections. In some cases, a device was not suitable, in which case it was retrieved and at least one other device was tried before final device placement using catheter techniques.
The device discs and their relationships with the neighbouring structures were carefully studied, including the device straddling the aorta and touching the atrial free wall. The latter was defined as either the left or right disc touching the atrial free wall in any direction according to TTE views. Multiple views of the atrial free wall were captured, including: 1) the parasternal short-axis view (posterior and inferior), 2) the four-chamber view or atypical four-chamber view (posterior and superior), and 3) the subcostal view (superior and inferior).
ASSESSMENT OF PERICARDIAL EFFUSION BY TTE
PE was quantified using the multiple views obtained with TTE: (1) position: localised or circumferential; (2) quantification: trivial (appeared only during the systolic cycle and was <5 mm), mild (during the diastolic cycle and was <10 mm), moderate (10-20 mm and was usually circumferential), large (>20 mm)14; (3) acoustical density: echolucent or fibrinous strands were present15.
FOLLOW-UP
All the patients were required to be hospitalised at the time of PE identification. The patients were discharged if they were in stable condition (mild PE with no increment, no new-onset arrhythmia, being stable haemodynamically, and device position being correct). The median time of hospital stay was four days. All patients were required to appear for one, three, and six-month outpatient follow-up evaluations. Additional follow-up was not required unless there was a specific problem or complaint.
STATISTICAL ANALYSIS
Continuous variables were described as the mean±SD and categorical variables as the number (percentage). For comparisons of continuous variables, the independent samples t-test was used. The χ2 test was used with two-tailed p-values for comparing categorical variables. Univariate and multivariate logistic regression were used to evaluate factors associated with the risk of new-onset PE. To avoid collinearity, separate models were constructed for device size and total defect size in multivariate analysis, to which we added patient age, device touching the atrial free wall, female sex, mean pulmonary artery pressure, right ventricular diameter. The odds ratio (OR) and its 95% confidence interval (CI) were calculated. Receiver operating characteristic (ROC) curves were generated for independent indices to obtain areas under the curve (AUC) and cut-offs. Analyses were performed with SPSS software, Version 20.0 (IBM Corp., Armonk, NY, USA). A value of p<0.05 was considered to indicate statistical significance.
Results
STUDY POPULATION
Of the remaining 2,652 patients (without pre-existing PE or cardiac perforation or erosion), 2,439 (92%) completed the one-month evaluation, and 2,294 (86.5%) and 2,119 (79.9%) patients completed the three and six-month evaluations, respectively. Finally, there were 50 patients with new-onset PE that appeared during the procedure or follow-up (PE+ group) and 2,602 patients without PE during the procedure or follow-up (PE- group).
THE OTHER RESULTS
A patient who was diagnosed with wire perforation (right atrium) was excluded. The patient exhibited moderate PE four hours after transcatheter closure of ASD. He suffered chest pain, fever (four days), atrial fibrillation, and hypotension. He underwent pericardiocentesis, and pericardial tamponade was alleviated after drainage of 445 ml of venous blood.
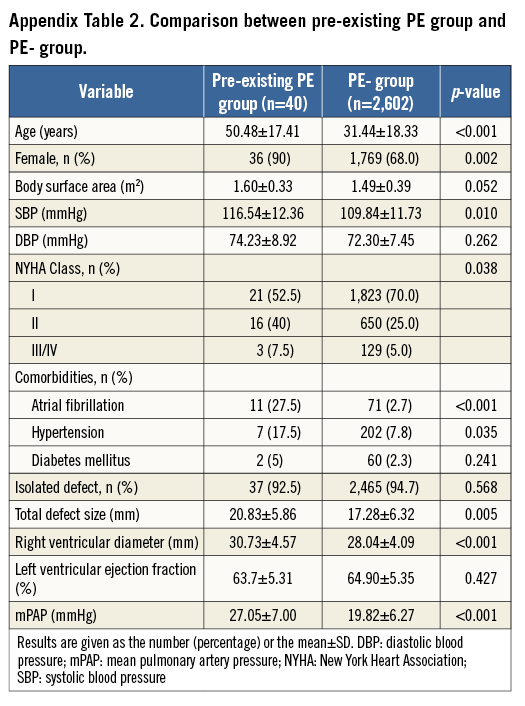
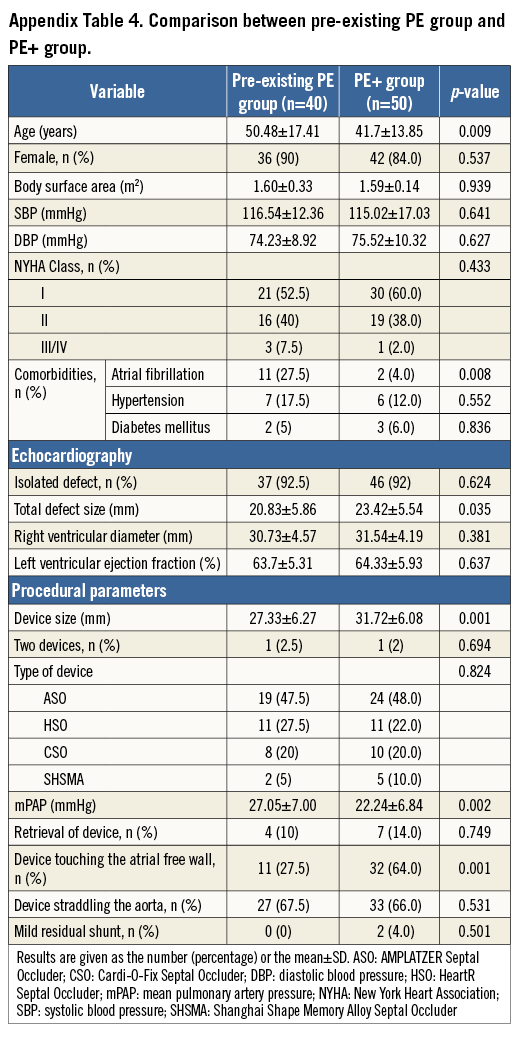
Forty patients were diagnosed with pre-existing PE (eight had moderate and 32 mild PE). There was a 1.5% incidence of pre-existing PE in the present study. No patient had hypoalbuminaemia, autoimmune disease, or renal abnormalities. Compared with 2,602 patients without PE, the strongest factor independently predicting pre-existing PE was atrial fibrillation. Compared with the PE+ group, the pre-existing PE patients were more likely to have atrial fibrillation, to be older, and to have a higher mean pulmonary artery pressure, even with smaller defect and device size (Appendix Table 2-Appendix Table 4). Moreover, fewer devices tended to touch the atrial free wall in the pre-existing PE group. Therefore, the 40 pre-existing PEs were excluded as there was no new-onset PE after ASD closure.
INCIDENCE AND OUTCOME OF PE
There was a 1.9% incidence (n=50) of new-onset PE that appeared during the procedure or follow-up. The incidence of PE with each device was 0.9%, 0.41%, 0.38%, 0.19% for the AMPLATZER Septal Occluder, HeartR Septal Occluder, Cardi-O-Fix Septal Occluder and Shanghai Shape Memory Alloy Septal Occluder (SHSMA), respectively. In all, 37 patients developed new-onset PE immediately after implantation of the device, 12 within one month, and one within three months. All cases of PE (n=50) diminished spontaneously (43 completely resolved, seven remained trivial and stable). The median time to relief (resolved or trivial) was 15 days. Figure 1 shows a patient who developed new-onset PE during the procedure which resolved spontaneously.
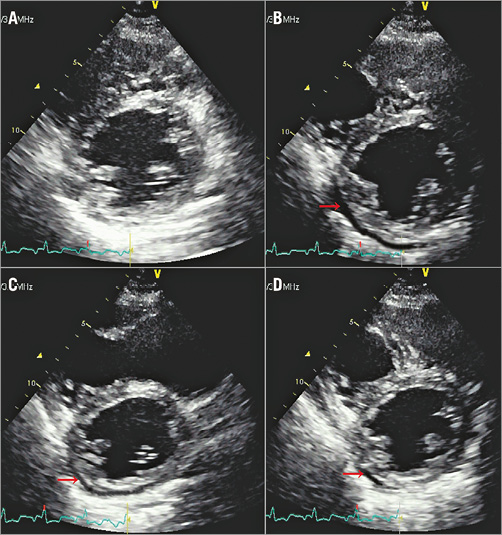
Figure 1. Transthoracic echocardiographic view of pericardial effusion (PE, red arrow) before, during, and after the procedure. A) Before procedure. B) During release of the occluder, PE was mild (5 mm). C) One hour later, the PE had decreased to 3 mm spontaneously. D) The next day, the PE was trivial.
ECHOCARDIOGRAPHIC CHARACTERISTICS OF PE
All cases of PE (n=50) evaluated by TTE were mild (5.1±1.9 mm) and homogeneously echolucent. There were 42 cases in the pericardial cavity of the posterior left ventricle or atrioventricular groove. Eight cases were in the pericardial cavity of the right atrium.
CLINICAL AND LABORATORY FEATURES OF THE PE+ GROUP
The patients with PE (n=50) were asymptomatic, haemodynamically stable, and experienced no new-onset arrhythmias during the procedure or the follow-up. None had autoimmune disease, allergies, or renal or hepatic abnormalities. The patients with PE had normal serum albumin (42.1±3.3 g/L) and serum creatinine (72.9±15.6 μmol/L). The white blood count (7.7±3.4×109/L) with normal neutrophil and eosinophil percentages was normal in 49 patients. In the other patient, the eosinophil count was 8.6% (laboratory normal range 0.4-8.0%). All data were acquired at the time of PE identification.
CLINICAL AND PROCEDURAL COMPARISON BETWEEN THE PE- AND PE+ GROUPS
Of the total number of patients (n=2,652), 706 (26.6%) were less than 18 years of age, 1,779 (67.1%) ranged from 18 to 60 years of age, and 167 (6.3%) were more than 60 years of age. Clinical characteristics are compared between the PE+ group (n=50) and the PE- group (n=2,602) in Table 1. Compared with the PE- group, the PE+ patients were older, more likely to be female, and had a larger body surface area. Other clinical characteristics were similar between the two groups.
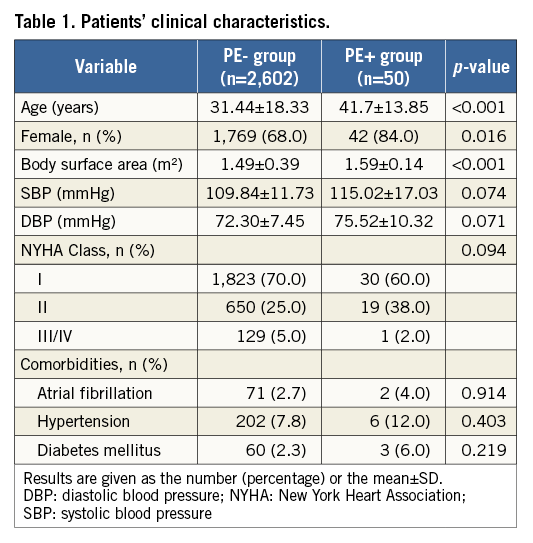
During the procedure, the PE+ group was found to have a larger defect size, device size, right ventricular diameter, and mean pulmonary artery pressure compared with the PE- group. In particular, more devices tended to touch the atrial free wall in the PE+ group but showed no significance as regards straddling the aorta. The type of device had no significant effect (Table 2).
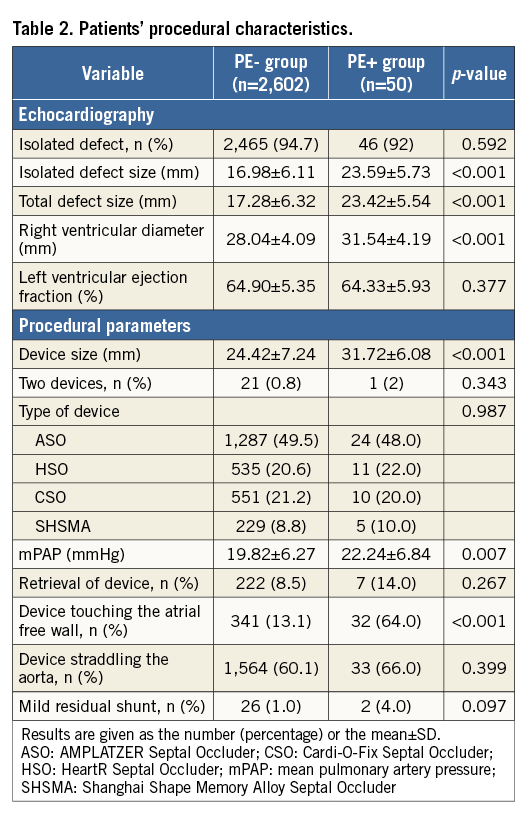
INDEPENDENT PREDICTORS OF ASYMPTOMATIC PE
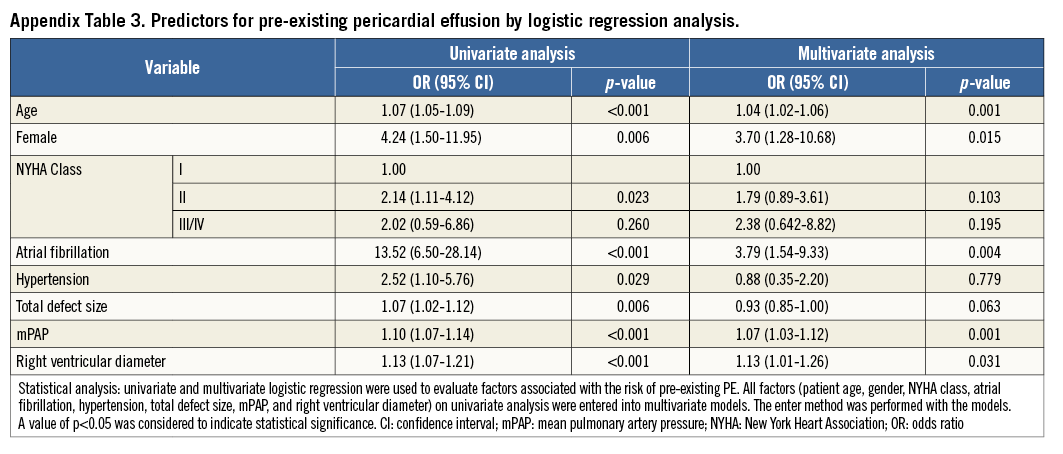
Logistic regression was employed to determine the factors that predict asymptomatic PE. Univariate analysis showed that the device touching the atrial free wall, total defect size, device size, mean pulmonary artery pressure, right ventricular diameter, patient age, and female sex were significant factors (Table 3). The significant factors were therefore entered into the multivariate analysis using the enter method (Table 4). To avoid collinearity, separate models were constructed for device size and total defect size (R2=0.22 in model 1, R2=0.21 in model 2). After analysis, the device touching the atrial free wall, device size, patient age, and total defect size remained independent predictors. ROC curves were constructed to define AUC and optimal cut-offs for asymptomatic PE. Areas under the curve were 0.78 (p<0.001), 0.66 (p<0.001) and 0.77 (p<0.001) for device size, patient age, and total defect size, respectively. The cut-off value for the device size was >27 mm, which offered 82% sensitivity and 63% specificity. The cut-off value for patient age was >39.5 years, which showed 54% sensitivity and 63% specificity. The cut-off value for total defect size was >18.5 mm, which offered 80% sensitivity and 61% specificity (Figure 2).
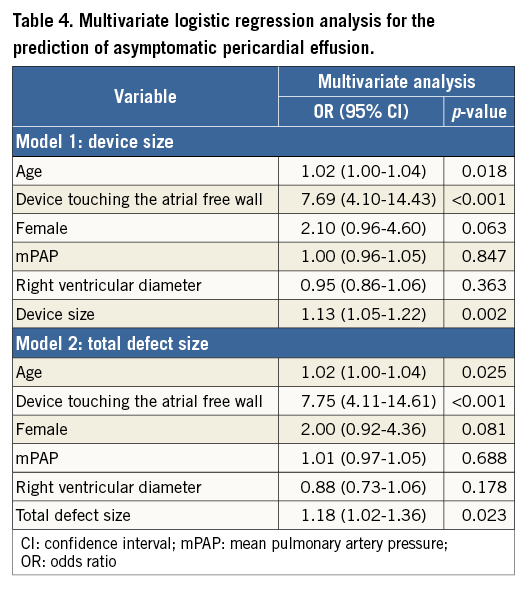
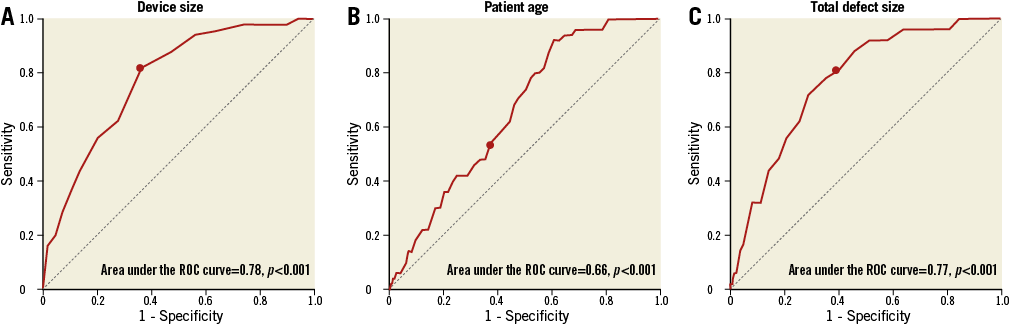
Figure 2. Receiver operating characteristic (ROC) curve of the ability of device size, age and total defect size to predict asymptomatic pericardial effusion. Areas under the curve were 0.78 (p<0.001), 0.66 (p<0.001), and 0.77 (p<0.001) for device size (A), patient age (B), and total defect size (C), respectively (cut-offs, dark dot). A device size >27 mm offered 82% sensitivity and 63% specificity; patient age >39.5 years showed 54% sensitivity and 63% specificity; total defect size >18.5 mm offered 80% sensitivity and 61% specificity.
Discussion
Clinically significant PE related to transcatheter closure of an ASD has been extensively described in previous studies, with the aetiology ranging from cardiac perforation to device erosion4,16. Few data, however, are available regarding asymptomatic PE unrelated to cardiac perforation or erosion. To the best of our knowledge, the present study is the first to report the incidence, natural history, clinical features, and predictors of asymptomatic PE.
This new type of PE is not due to cardiac perforation, device erosion, or other causes of pericarditis (bacterial, uraemic, or autoimmune). It occurs slowly, is of mild volume, is asymptomatic, and diminishes spontaneously. It may be described as reactive PE.
There were substantial differences between pre-existing and reactive PE involving aetiology and outcome. With pre-existing PE, the patients were more likely to have atrial fibrillation compared with control. Alternatively, there was an obvious effect of percutaneous ASD closure on patients with reactive PE: 74% (37/50) of patients developed new-onset PE on the day of the procedure. This was as opposed to pre-existing PE, in which no patient had an increment of PE after ASD closure. In particular, more devices tended to touch the atrial free wall in the reactive PE group, which could be a sign of procedural effect. Last but not least, the outcome of reactive PE was benign with spontaneous relief in all patients. However, with mild to moderate pre-existing PE, Reddy et al17 speculated that, if septal defects were left untreated, the effusions may accumulate over time because of chronic volume overload, which may lead to more PEs.
Our interventional procedure is comparable to the international protocol except for the TTE algorithm. In the determination of defect measurements, defect sizes by TTE were smaller than those of TEE or three-dimensional TEE measurements18, similar to our results included in the supplementary data. The selection of device size tended to be 6-8 mm above the defect size in our study. There was no procedure-related death, and the rate of major complications was 0.59% (14/2,392) with the strategy19, which was not higher than that of previous algorithms20. The incidence of reactive PE showed no significance between the present and previous study (1.9% versus 1.6%, p=0.973)6. The type of algorithm had no significant effect.
The AMPLATZER Septal Occluder is the most common dedicated device for percutaneous ASD closure. However, the Cardi-O-Fix Septal Occluder, HeartR Septal Occluder, and SHSMA Septal Occluder have been verified to be as safe and effective as the AMPLATZER Septal Occluder21-23. The last three devices appear to be widely used in China because of their relatively low cost. Our study demonstrated that the type of device did not have any effect in either the PE+ or the PE- group.
We postulated that the device touching the atrial free wall, device size, total defect size, and patient age might all play a role in the pathogenesis of asymptomatic PE. The device touching the atrial free wall is the strongest independent predictor. Previous findings suggested that local tissue inflammation appeared a few days after device implantation, with the response inducing entry of an exudate into the pericardial space24. The device straddling the aorta is not a significant factor, possibly because there is no pericardial tissue encompassing the root of the aorta. One study showed that the right ventricular size decreased over time25, enlarging the area between the device and the atrium, possibly inducing reactive PE during follow-up. Furthermore, a previous study showed that segmental systolic left ventricular function was normal in patients with small or medium-size devices. Conversely, left ventricular function was significantly impaired in patients who underwent implantation of a large device26. Also, in middle-aged adults with pre-existing decreased left ventricular compliance, the acute increase in preload associated with defect closure could lead to worsening left ventricular function27.
CLINICAL IMPLICATIONS
We undertook quantitative TTE in patients with percutaneous ASD closure. This adds to our understanding of new-onset PE related to percutaneous ASD closure. When PE is a complication of an intracardiac procedure, it is usually considered to be the result of cardiac perforation. The diagnosis and differential diagnosis of PE depend on several factors: (1) the symptoms and haemodynamic status of patients4,28; (2) the cardiac rhythm (blood and blood products are quite irritating, and the sinoatrial node is an epicardial structure so PE with blood may give rise to some arrhythmias29); (3) the echocardiographic and radiological assessment. The speed of accumulation, level, and echo density may be different for cardiac perforation and reactive PE. Reactive PE is mild, homogeneously echolucent, and diminishes spontaneously. Detection of fibrin strands implies the presence of haemorrhagic PE15.
We demonstrated that age >39.5 years, device size >27 mm, total defect size >18.5 mm or the device touching the atrial free wall are risk factors for reactive PE.
The aetiology of PE during intracardiac procedures should be identified. Reactive PE should be considered in patients with an unexplained cause, adding to our understanding of the aetiology of new-onset PE. Pericardiocentesis can be life-saving with cardiac tamponade but may be unnecessary with reactive PE. It leads to different therapeutic strategies. Our new finding gives us a more comprehensive understanding of the diagnosis and differential diagnosis of new-onset PE. It also expands our understanding of the relationship between device selection and cardiac anatomy.
Limitations
This was a retrospective study, which is less comparable to a prospective study. Our study, limited by the TTE algorithm, albeit demonstrating a new type of PE with percutaneous ASD closure, was not validated with a TEE or balloon algorithm. Longitudinal studies may be able to clarify this further using international protocol. Although the device touching the atrial free wall was the most independent predictor of reactive PE, the mechanism was not validated with sufficient evidence.
Conclusions
The incidence of asymptomatic PE unrelated to cardiac perforation or erosion was low, and the natural history was self-limited. However, we identified early or delayed onset, mild volume, homogeneous echolucency, and being asymptomatic as important features of this benign condition. Our study demonstrated for the first time the characteristics of asymptomatic PE determined by TTE. The factor of the device touching the atrial free wall may be important in the pathogenesis of asymptomatic PE.
| Impact on daily practice Pericardial effusion without obvious periprocedural complications (e.g., cardiac perforation, device erosion) may occur after transcatheter closure of secundum atrial septal defects. The pericardial effusion was benign and self-limited. Early or delayed onset, mild volume, homogeneous echolucency, and being asymptomatic constitute the important features of this benign condition. |
Acknowledgements
The authors thank the staff of the Radiology Department, Fuwai Hospital, for their help in clinical data acquisition.
Conflict of interest statement
The authors have no conflicts of interest to declare.