
Abstract
Aims: The aim of this study was to describe the incidence, mechanisms, management and outcomes of intracardiac shunts (ICS) following TAVI.
Methods and results: This was a multicentre registry across 10 centres aimed at gathering all cases of ICS (1.1%) including infection-related (IRICS, 0.3%) or aseptic (AICS, 0.8%) shunts. Patients presented porcelain aorta (24% vs. 6.8%, p=0.024) and had been treated with predilation (88% vs. 68.5%, p=0.037) or post-dilation (59.1% vs. 19.3%, p<0.001) more often. Median time from intervention to diagnosis of ICS was 10 days (IQR: 2-108), being longer for IRICS (171 [63-249] vs. 3 [1-12] days, p=0.002). Interventricular septum (55.6%) and anterior mitral leaflet (57.2%) were the most common locations for AICS and IRICS, respectively. Most patients (76%) developed heart failure but 64% were medically managed. Seven patients (38.9%) underwent percutaneous closure of AICS. The in-hospital mortality rate was 44% (IRICS 100%, AICS 27.8%) compared to global TAVI recipients (8.1%, p<0.001). At one-year follow-up, 76% of the patients had died. ICS, logistic EuroSCORE, and moderate-severe residual aortic regurgitation were independent predictors of death.
Conclusions: Post-TAVI ICS are an uncommon complication independently associated with high early mortality. Currently, most therapeutic alternatives yield poor results but percutaneous closure of AICS was feasible and is a promising alternative.
Abbreviations
AICS: aseptic intracardiac shunts
CT: computed tomography
ICS: intracardiac shunts
IRICS: infection-related intracardiac shunts
NYHA: New York Heart Association
TAVI: transcatheter aortic valve implantation
Introduction
As the experience with transcatheter aortic valve implantation (TAVI) increases worldwide1,2, interest in risk factors and treatment for less common complications is growing. Several registries have been essential to improve the outcomes of patients at risk for some of these worrisome complications3-7; however, some life-threatening events remain barely explored.
The development of intracardiac shunts (ICS) after TAVI is a rare complication, often diagnosed days after TAVI, which typically requires prompt recognition and treatment to prevent death, as has been suggested previously8. The mechanism of this complication can be mechanical compression of highly calcified structures, inadvertent perforation by guidewires or sheaths, or local infection8. Strategies to prevent this problem, detect it earlier, and treat before progression are not systematically applied but could radically improve the prognosis of patients with ICS.
Therefore, the aim of this study was to evaluate the incidence, predisposing conditions, management, and outcomes of patients who develop ICS, of both infective and non-infective aetiology, following TAVI.
Methods
All cases of ICS irrespective of their aetiology were collected retrospectively across 10 centres from January 2009 to December 2016. ICS were defined as any shunts occurring between two cardiac chambers or great vessels (pulmonary artery and aorta), in relation to the use of a TAVI device. This was the only inclusion criterion; no exclusion criteria were pre-specified. Contentious cases, including those with mitral leaflet perforation, were discussed and finally included according to the pre-specified definition of ICS. Data gathered included the main baseline, echocardiographic, computed tomography (CT), procedural, and follow-up characteristics.
In addition, all centres were asked to provide main patient-level data of the entire population who underwent TAVI within the inclusion period. All centres had a dedicated TAVI database where all cases were prospectively recorded, and a systematic follow-up was performed through clinical visits and/or telephone contact. Data collected included age, sex, logistic EuroSCORE, STS score, comorbidities, previous coronary artery disease, peripheral artery disease, echocardiographic and CT parameters (including amount and distribution of calcium, aortic annulus dimensions), and procedural details. In case of suspected infective endocarditis, the potential source of infection, bacterial cause, initial symptoms, antibiotic treatment and its duration were recorded. This registry was approved by local ethics committees.
SPECIFIC PROCEDURAL AND ICS FEATURES
Data on the approach, type and size of prosthesis, predilation and post-dilation, haemodynamic instability and any procedure-related complication defined according to Valve Academic Research Consortium (VARC)-2 criteria9 were gathered. The main details of ICS included clinical presentation, location, size of shunts as assessed by echocardiography, timing after TAVI, treatment performed, and one-year follow-up.
STATISTICAL ANALYSIS
Data are expressed as absolute frequency and percentage in case of qualitative variables. Quantitative variables are described as mean (SD) or median (25th-75th interquartile range [IQR]) depending on variable distribution. Group comparisons were analysed using the Student’s t-test or its non-parametric equivalent, the Mann-Whitney U test, for continuous variables, and the chi-square test or Fisher’s exact test for categorical variables. Survival analysis by the Kaplan-Meier method was used to determine differences according to the subgroup of patients with or without ICS. Multivariate analysis through Cox regression was used to evaluate independent predictors of mortality in the global study population. Long-term follow-up was available in all centres but those with more than 5% of data missing at follow-up were excluded from the Kaplan-Meier analysis. Statistical significance was defined as p-value <0.05. All analyses were conducted using the software package SPSS Statistics, Version 23.0 (IBM Corp., Armonk, NY, USA).
Results
BASELINE CHARACTERISTICS OF INTRACARDIAC SHUNTS
The mean age of the study population (N=2,239) was 80.3±7.4 years and 51.6% were female. A total of 25 patients (1.1%) developed ICS within a period of 10 days (IQR: 2-108) after TAVI. The increase in the number of TAVI procedures across participating institutions was exponential over time, whereas the number of ICS presented a linear increase, suggesting a decrease in the proportion of patients with this problem (Supplementary Figure 1). The clinical, echocardiographic and CT baseline characteristics are summarised in Table 1. Most patients with ICS were female (60%), with lower surgical risk according to logistic EuroSCORE (9.25% [IQR: 4.6-16.6] vs. 17.2% [IQR: 10.7-26.5], p<0.001), and higher mean gradients (55.8±19.1 mmHg vs. 46±16.7 mmHg, p=0.004). A diagnosis of porcelain aorta was made more often in patients with ICS (24% vs. 6.8%, p=0.024) and 12% had prior chest wall radiation therapy for cancer.
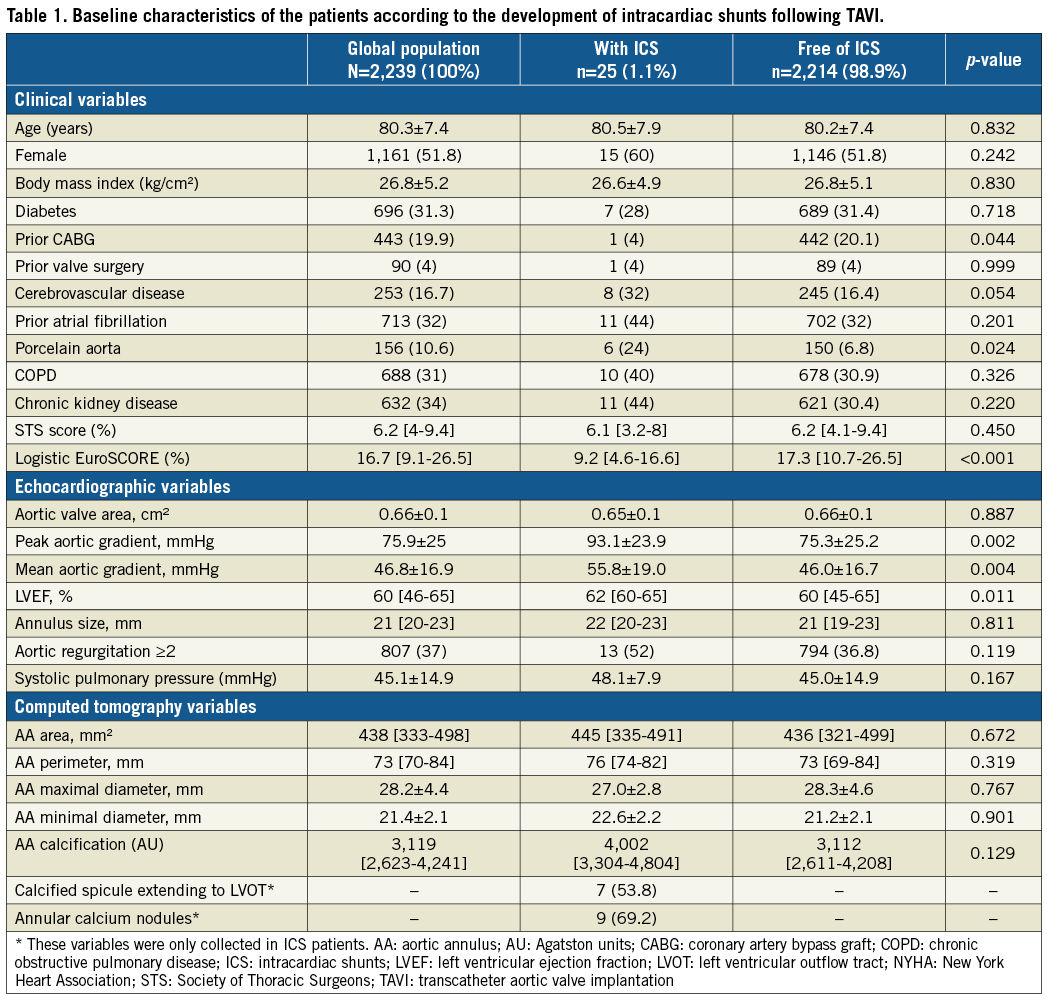
CT findings in patients with ulterior development of ICS included aortic valve calcium score of 4,002 (IQR: 3,304-4,804) Agatston units (AU) with nine patients (69.2%) showing calcium nodules in the aortic leaflets, and seven patients (53.8%) asymmetric calcified spicules extending to the left ventricular outflow tract.
PROCEDURAL AND IN-HOSPITAL OUTCOMES
The procedural characteristics of the study population are shown in Table 2. Of the 25 patients with ICS, the mechanism was direct compression of calcium (aseptic intracardiac shunt [AICS]) in 18 cases (72%, incidence 0.8%) and infective fistulisation (infection-related intracardiac shunt [IRICS]) in seven patients (28%, incidence 0.3%). No cases were caused by catheters or guidewires as described elsewhere8. Overall, the median time from the intervention to the diagnosis of ICS was 10 days (IQR: 2-108).
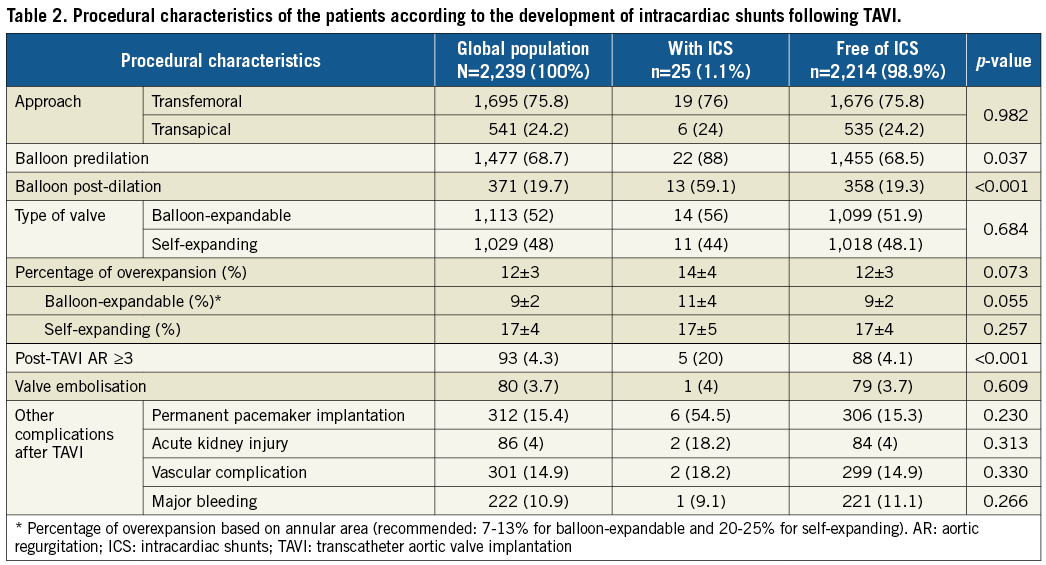
As compared to the global TAVI recipients, those patients with ICS more often received balloon predilation (88% vs. 68.5%, p=0.037) and post-dilation (59.1% vs. 19.3%, p<0.001). A trend to greater overexpansion was detected in patients treated with balloon-expandable prostheses with shunts compared to those without. On the other hand, although absolute overexpansion was greater with self-expanding devices, no differences were found in those with shunts (Table 2). No other differences regarding the approach, valve size, or use of balloon-expandable devices (56% vs. 52%, p=0.684) were found. Patients developing ICS more commonly presented residual moderate or severe aortic regurgitation (20% vs. 4.1%, p<0.001) and had a high rate of other concomitant TAVI-related complications, including atrioventricular block requiring permanent pacemaker implantation in 54.5%.
In-hospital symptoms related to the development of ICS occurred in 19 patients (76%), whereas six patients (24%) remained asymptomatic. Heart failure was the most common initial symptom (76%) and fever was present in most patients developing IRICS (85.7%). In-hospital mortality occurred in 11 of the patients with ICS (44%) and was significantly higher than among the global TAVI recipients (vs. 8.1%, p<0.001) (Figure 1).
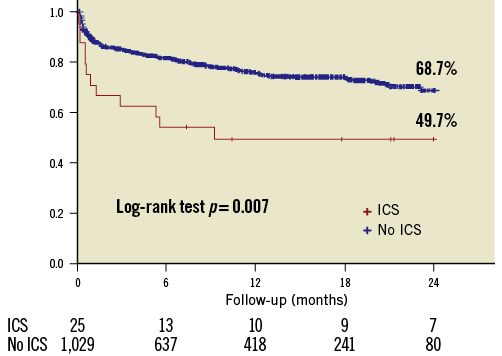
Figure 1. Two-year follow-up survival curves for the global population of TAVI recipients and for those who developed intracardiac shunts.
MAIN FEATURES OF INTRACARDIAC SHUNT ACCORDING TO THE MECHANISM
The location of ICS according to their aetiology is depicted in Figure 2 and summarised in Table 3. Ventricular septal defect was the most frequent location of AICS (55.6%), whereas anterior mitral leaflet perforation shunting blood from left ventricle to left atrium was the most common one in patients developing IRICS (57.2%). Aortic root-to-left ventricle and aortic root-to-right atrium fistula were diagnosed with either AICS or IRICS. Although specific predisposing conditions could not be identified for each subtype of intracardiac shunt given the low number of cases, the presence of bulky calcification extending to the outflow tract was described in all cases of ventricular septal defect.
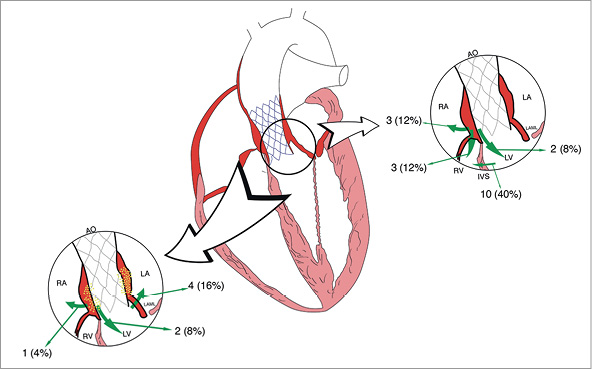
Figure 2. Schematic location of intracardiac shunts following TAVI according to aetiology. Left: infection-related intracardiac shunts (IRICS). Right: aseptic intracardiac shunts (AICS).
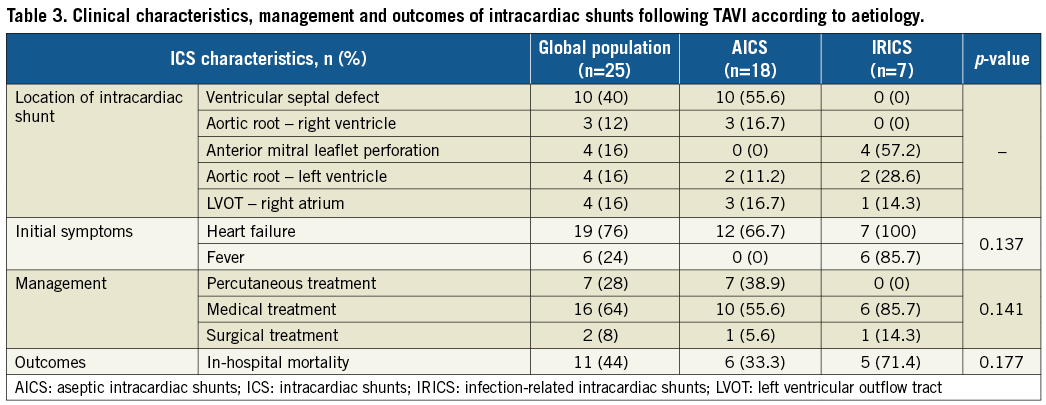
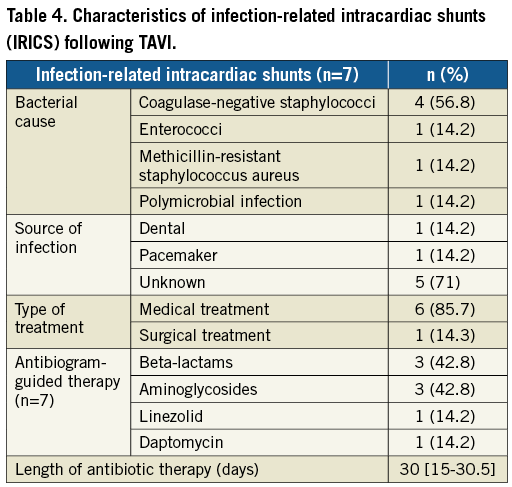
The diagnosis of IRICS was made significantly later than in patients with AICS (171 [63-249] vs. 3 [1-12] days, p=0.002), and the size of the shunt could be determined only in AICS cases (4.5 [IQR: 3-5.5] mm) due to anfractuosity of the fistula in IRICS. Aetiology of the infection - when present - is summarised in Table 4. Coagulase-negative staphylococci were the most common bacterial cause and sources of infection were identified in only two patients, with all IRICS presenting definitive infective endocarditis criteria. From a total of 42 cases of endocarditis (1.87% of the study population), 16.7% had a fistula. The main aetiology (coagulase-negative staphylococci in 54.8%), chronology (“early” endocarditis in 92.9%), and one-year mortality (69%) of patients suffering from infective endocarditis were similar to those of patients with IRICS.
MANAGEMENT AND OUTCOMES OF INTRACARDIAC SHUNT ACCORDING TO AETIOLOGY
Treatment options (Figure 3) included open surgery in two patients (8%), percutaneous closure of the shunt in seven patients (28%), and conservative treatment in the remaining 16 patients (64%). All percutaneous closure attempts were performed in patients with AICS. They were all symptomatic and the mean Qp/Qs was 2.3±0.3 as opposed to 1.86±0.3 (p=0.01) in the cases medically treated. Selected devices included the Nit-Occlud® PDA (pfm medical ag, Cologne, Germany) in three cases, AMPLATZER Vascular Plug II (St. Jude Medical, St. Paul, MN, USA) in two cases, Flipper® PDA Closure Detachable Coil (Cook Medical, Bloomington, IN, USA) in one case, and AMPLATZER ASD Occluder (St. Jude Medical) in one other case. Successful closure of the shunt was achieved in 100% of the attempts. Four patients (57.1%) died during the index hospital stay and the other three within the first year. In 42.8% of the cases the cause of death was progression of heart failure. One other patient with an aseptic ventricular septal defect causing heart failure underwent surgical repair seven days after TAVI. Direct suture and implantation of a bioprosthesis was successful without events in the follow-up. Finally, the mortality rate of the 10 cases which were medically treated was 50% at a mean follow-up of 5.3 [IQR: 1.3-29.3] months from the diagnosis, leading to a final one-year mortality rate in the AICS group of 66.7%.
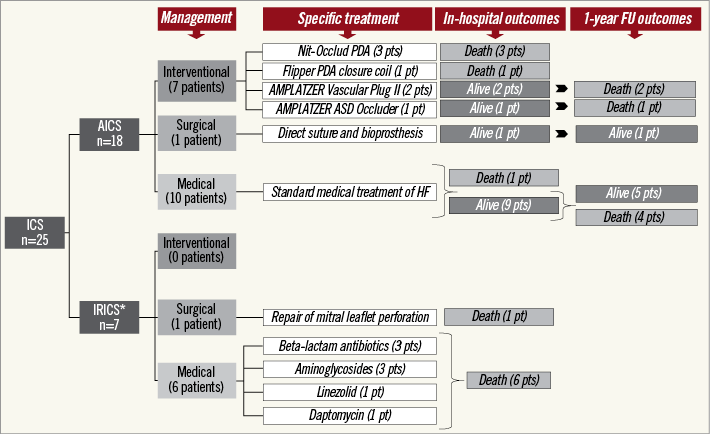
Figure 3. Flow chart summarising the management and outcomes of patients suffering from intracardiac shunts following TAVI. *All patients in the IRICS group received antibiogram-guided therapy (either one or more drugs). Only one of them also underwent surgery.
All patients with IRICS died during the index admission. Six of the seven patients with IRICS (85.7%) were medically treated including specific antibiotic regimes during a median time of 30 days (IQR: 15-30.5). Surgical repair of the valve was performed in one case with mitral leaflet perforation but perioperative infection led to the death of the patient. Multiorgan failure (50%), heart failure (33.3%), and septic shock (16.7%) were the causes of death in IRICS patients.
Thus, at two years of follow-up from the TAVI procedure, mortality was significantly higher in patients with ICS (50.3% vs. 31.7%, p=0.007) (Supplementary Table 1). ICS, logistic EuroSCORE, and moderate-severe residual aortic regurgitation amongst others were independent predictors of death.
Discussion
ICS are an infrequent, life-threatening, and preventable complication following TAVI. Mechanisms are probably shared with other uncommon but severe complications in the field of TAVI such as annulus rupture or coronary obstruction, representing one of the main pitfalls to the extension of this technology to patients with lower risk. There are several key findings from this research. 1) ICS were an independent predictor of mortality in patients treated with TAVI. 2) AICS may be partially preventable by more accurate valve sizing and device selection, reducing the need for post-dilation and overexpansion in patients with adverse calcium amount and distribution or those with prior chest radiation. 3) This problem did not require emergent management in any case. Hence, best treatment can be discussed and carefully planned in this setting to overcome the numerous caveats of the current therapeutic alternatives. Surgery was discarded in most patients, probably due to very high risk and a high prevalence of porcelain aorta, but medical and percutaneous alternatives in symptomatic patients also yielded poor results with in-hospital mortality rates of 29.4% and 66.7%, respectively. The suboptimal midterm outcomes reported in patients treated percutaneously contrast with positive results in previous reports, highlighting the current lack of reproducibility of this approach8. However, percutaneous treatment is probably the most promising alternative for AICS, suggesting the need for thorough research to develop dedicated devices and three-dimensional models helpful for procedural planning10.
MECHANISM OF ICS
Some authors have suggested that balloon-expandable valves might be related to a higher risk of ICS due to forceful expansion in contrast to self-expanding devices which are more adaptable to the landing zone8. Several aspects from our research support this hypothesis. First, as recommended, greater overexpansion was used for self-expanding valves but no differences in the percentage of overexpansion were detected with this device in patients with ICS. On the other hand, in patients treated with balloon-expandable prostheses, there was a trend to greater overexpansion as compared to those free of this complication (Table 2). Balancing the risk of paravalvular regurgitation versus development of ICS or annular rupture is a major responsibility of the TAVI team that should be addressed by adequate device selection and precise determination of the overexpansion being aimed at before the procedure. Also, if anterior mitral leaflet perforation cases were excluded, patients with balloon-expandable devices presented twice the incidence of ICS (1.26%) as compared to self-expanding ones (0.68%). Conversely, as we advance the system deeper within the left ventricle, the outflow tract frequently tapers down and it may be much smaller at the site of the valve landing zone. In this regard, deeper implantation can be performed more often with self-expanding systems and might have an impact on the rate of interventricular shunts.
Regarding IRICS, the relatively high incidence of this complication (one out of every six cases of infective endocarditis) suggests that TAVI may more easily result in complications involving a fistula in this context due to the technical features of the devices, with a large stent frame extending to the ascending aorta and deep into the outflow tract. An alternative controversial hypothesis is that a fistula may occur first, acting as the substrate for infective endocarditis7,11-14. Clarifying this aspect is of the utmost importance as the management is radically different and conditions the prognosis. Moreover, diagnosis of IRICS is more challenging and, as a result, the rate of this complication may be underreported. As a general recommendation, a systematic echocardiographic evaluation should be performed in all patients harbouring a TAVI device who present a progression or develop new symptoms of heart failure, and attention should be paid to detecting subtle signs of fistula or infective endocarditis.
MANAGEMENT AND OUTCOMES OF ICS
The development of ICS was independently associated with higher mortality but no therapeutic strategy has yet been demonstrated to reduce the risk of death effectively once the complication has occurred. The growing experience in the field of percutaneous closure of paravalvular leaks might be useful for the management of ICS in symptomatic patients15. A specific concern in this setting is the risk of interaction with the TAVI device leading to prosthesis malfunction8. Selected low-profile devices with double-disc systems or smaller and flexible coils can be useful. Percutaneous closure of AICS was considered feasible in 38.9% of the patients and the technical success rate was 71.4% (lower than periprosthetic leak closure in alternative scenarios)15. Nevertheless, the in-hospital mortality remained high (57.1%). An earlier attempt to close the shunt in less symptomatic patients or in case of a high Qp/Qs ratio may help to diminish the mortality8. Conservative treatment, albeit a potential option in asymptomatic patients with low values of Qp/Qs ratio, should be accompanied by close follow-up to prevent rapid deterioration. However, in patients with infective endocarditis, the extremely poor outcomes with conservative management should lead to reconsideration of surgery as the only potentially life-saving alternative.
Limitations
The main limitation of the study was the relatively low number of patients with ICS as a result of its low incidence. This precluded multivariate analysis to determine the independent predictors of this complication. Small shunts in asymptomatic patients may not have been detected due to the lack of central imaging analysis. Finally, infective and non-infective shunts present radically different mechanisms, management, and prognosis but both were included, though separately described, given the poor knowledge in this field and the challenging differential diagnosis in some cases.
Conclusions
ICS following TAVI are an uncommon complication with 3/4 due to mechanical compression of calcium and 1/4 related to infective endocarditis. Mortality was >50% at one year of follow-up and the prognosis was especially poor for those patients who presented infection or symptoms of heart failure. Medical treatment in symptomatic patients was associated with very high mortality whereas percutaneous closure of AICS was feasible but of limited efficacy, requiring further investigation to determine whether earlier attempts or specific devices might improve outcomes.
| Impact on daily practice Intracardiac shunts (ICS) are a rare complication of TAVI procedures with a relevant impact in terms of symptoms and are independently associated with early mortality. Most of the ICS were the consequence of mechanical compression of calcium due to its extension towards the left ventricular outflow tract but also as a result of prosthesis overexpansion. Also, a small proportion of the cases, with poorer prognosis, occurred due to infective endocarditis causing fistulisation. Current therapeutic alternatives yielded poor results, but percutaneous closure of aseptic shunts is a new and promising alternative. |
Acknowledgements
We acknowledge Pilar Jimenez-Quevedo, Valeriano Ruiz, José M. Hernández García, Bruno García del Blanco, Rosana Hernández-Antolin, Luisa Salido, Luis R. Goncalves-Ramirez, Pablo Catalá, Itziar Gómez, and Pablo Valladares for their contributions to this research.
Funding
P. Rojas has received support from Fundación Carolina-BBVA.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Table 1. Main predictors of follow-up mortality in the global TAVI population.
Supplementary Figure 1. Number of TAVI procedures, total number of intracardiac shunts, and aseptic shunts post TAVI.
To read the full content of this article, please download the PDF.

