Abstract
Aims: Transcatheter aortic valve implantation (TAVI) has recently developed into an accepted alternative to conventional surgery in high-risk patients. According to current data, post-TAVI prosthetic valve endocarditis (PVE) seems to occur very rarely.
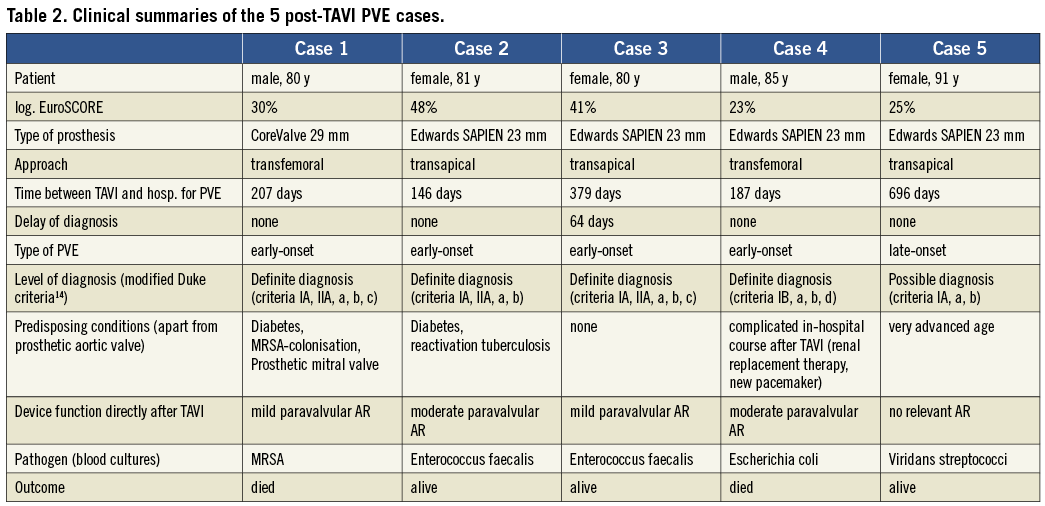
Methods and results: We followed the first 180 consecutive patients who underwent TAVI at our institution to assess safety and efficacy of the procedure. During follow-up (median, 319 days), PVE was seen more frequently than expected. By applying modified Duke criteria five cases could be confirmed (four early-onset and one late-onset PVE, four cases with “definite diagnosis” and one with “possible diagnosis”) representing an estimated PVE incidence of 3.4% at one year. Two patients died subsequently. Clinical summaries of all cases are reported and compared to previously published case reports.
Conclusions: According to our hypothesis, PVE might be particularly difficult to diagnose after TAVI, whereas TAVI-specific elderly patients might be exceptionally vulnerable. There exists little experience of TEE interpretation in post-TAVI endocarditis which should possess unique characteristics regarding, e.g., valve dehiscence or abscess formation. Therefore, echocardiography as a diagnostic tool often remains initially inconclusive. Because of incongruence between prosthetic device and calcified native aortic valve, some degree of paravalvular leak is common after TAVI. These paravalvular leaks as a nidus for infection, advanced age and abundant comorbidities might predispose TAVI patients for infective endocarditis.
Introduction
Prosthetic valve endocarditis (PVE) is a very serious complication of valve replacement and occurs with an incidence of 0.3-1.2% per patient-year1. A subdivision into early-onset PVE (defined as occurring within one year of surgery) and late-onset PVE (beyond one year) is carried out because of significant differences in observed microbiological profiles2. Considerable high in-hospital mortality rates of up to 38% for early-onset PVE2 and 25% for late-onset PVE were reported3. Clearly, an early and accurate diagnosis is essential to a favourable outcome.
The technique of transcatheter aortic valve implantation (TAVI) has recently developed into an accepted alternative to conventional surgery in high-risk patients with symptomatic aortic stenosis4. In brief, either a balloon-expandable or a self-expandable stent with an attached pericardial valve is positioned within the native aortic annulus via transarterial or transapical approach. The delivery balloon is inflated to expand the valved stent, or the self-expandable stent is released, thereby pressing the cusps of the native aortic valve against the wall of the aortic root. Patients accepted for TAVI are usually characterised by advanced age (>75 years), numerous comorbidities and high surgical risk commonly estimated by scoring systems (i.e., logistic EuroSCORE). According to limited current data, post-TAVI endocarditis seems to be a very rare event5-11. In the present study, we will, however, report five cases of suspected PVE in patients treated with TAVI at our clinic, and discuss special characteristics of PVE in the context of TAVI.
Methods
We previously assessed the safety, efficacy and clinical benefit of TAVI in the first 180 consecutive patients undergoing this intervention at our institution between August 2008 and December 201012. In-hospital complications and mortality at 30 days were recorded according to VARC definitions13. Furthermore, all patients were followed by telephone contact in 2011 and questioned about clinical symptoms, further hospitalisations and cases of death. Following the patients’ or their relatives’ statements, general practitioners, cardiologists and other hospitals were contacted and medical documents were acquired to investigate the incidence of major adverse cardiovascular and cerebrovascular events (MACCE), of valve-related events as well as the causes of death during follow-up according to VARC definitions13. The diagnosis of suspected PVE during follow-up was verified by applying modified Duke criteria14 to these cases. A pathological examination of valvular tissue as the gold standard for the diagnosis of infective endocarditis or postmortem analyses could not be carried out.
Implantation procedures using the retrograde transfemoral and the antegrade transapical approach were performed as previously described15-17. Transfemoral procedures were performed in a hybrid room and transapical procedures in an operating room. Perioperative antibiotic prophylaxis with cefazoline was administered routinely to all patients.
The study was approved by the local ethics committee; written informed consent for the procedure itself, for the recording of procedure-related complications and for follow-up telephone interviews to determine the incidence of MACCE, valve-related events and deaths, were obtained from all patients before the TAVI procedure.
Survival analysis was performed by the Kaplan-Meier method, with patients counted as of the last date known alive. Survival proportions according to Kaplan-Meier analysis were reported for six months and one year.
Results
The TAVI procedure was performed in 180 severely symptomatic patients characterised by a mean age of 82±5 years and a high risk for conventional surgery estimated by a mean logistic EuroSCORE of 27±14%. Mortality at 30 days was 8.9% and in-hospital mortality 10%. The telephone follow-up in 2011 was 99.4% complete (median follow-up period of 319 days). Survival proportions at six months and 12 months according to Kaplan-Meier analysis were 82±2.9% and 72±3.6%, respectively. Detailed information on the patient cohort’s baseline characteristics, procedural characteristics, perioperative outcome and midterm morbidity and mortality have previously been published12.
At follow-up, 51 of the 180 patients (28.3%) had died. The causes of death occurring after discharge from the index hospitalisation (33 cases) were the following: unexplained sudden deaths (n=8); congestive heart failure (n=5); device endocarditis (n=2); stroke (n=1); pneumonia or other septicaemia (n=4); cancer (one patient); surgery after hip fracture (one patient); ileus of small intestine (one patient); old age and bad clinical condition (10 patients). Reasons for further hospitalisation during follow-up included (note: one patient could have more than one event): congestive heart failure (n=26), stroke (n=9), pneumonia or other septicaemia (n=7), myocardial infarction (n=4), complications of TAVI procedure occurring after first discharge (n=4), syncope requiring pacemaker implantation (n=3) and aortic valve-related events (two re-interventions, five cases of device endocarditis, two further cases of suspected but not confirmed device endocarditis).
In summary, mortality and rehospitalisation rates in our patients were mainly attributable to congestive heart failure, stroke and infectious diseases. Prosthetic valve endocarditis accounted for a significant proportion of hospitalisations. We observed five cases (including two deaths) of post-TAVI endocarditis during a median follow-up of 319 days in 180 consecutive patients treated with TAVI. After Kaplan-Meier analysis, this represents an estimated PVE incidence of 3.4% (95% CI: 0.08 to 6.7) at one year in our patient cohort. Regarding the incidence density rate, the person-time incidence rate was 2.99% per patient year.
In the following, we will describe in detail the clinical courses of the five cases of prosthetic valve endocarditis. Two of these cases were diagnosed and treated at our own university hospital, whereas the remaining three patients were admitted to local hospitals nearer to their places of residence.
Case reports
CASE 1
An 80-year-old male (preoperative logistic EuroSCORE 30%) underwent TAVI because of severe symptomatic aortic stenosis in October 2009. The patient had a history of myocardial infarction with papillary muscle rupture and acute severe mitral regurgitation necessitating coronary artery bypass grafting (CABG) and implantation of a prosthetic mitral valve (29 mm; St. Jude Medical, St. Paul, MN, USA) in April, 2008. Furthermore, he suffered from a moderately reduced left ventricular function, peripheral artery disease, renal failure and diabetes.
Routine screening on admission before TAVI revealed nasal colonisation with methicillin-resistant staphylococcus aureus (MRSA). A decolonisation regimen with mupirocin nasal ointment was carried out, and surveillance cultures were negative for MRSA. The transfemoral implantation of the CoreValve prosthesis (29 mm, CoreValve ReValving System; Medtronic Inc., Minneapolis, MN, USA) and the hospital stay after TAVI were uneventful.
Seven months later, the man was readmitted to a local hospital because of acute congestive heart failure and fever >39°C. Three separate blood cultures were positive for MRSA. Transoesophageal echocardiographic (TEE) images are shown in Figure 1A and Figure 1B. Oscillating intracardiac masses could be detected neither on the prosthetic mitral valve nor on the TAVI device. However, the CoreValve prosthesis showed a relevant dynamic paravalvular leak (see arrows) enlarging in diastole (Figure 1B) and thereby causing further deformation of the prosthesis. Furthermore, diffuse thickening of the aortic root (Figure 1A) and a discontinuity in the native aortic annulus (Figure 1B) were present. Due to these findings, the treating physicians made the diagnosis of an aortic root abscess with a pseudoaneurysm. A direct postoperative TEE for matters of image comparison was not performed.
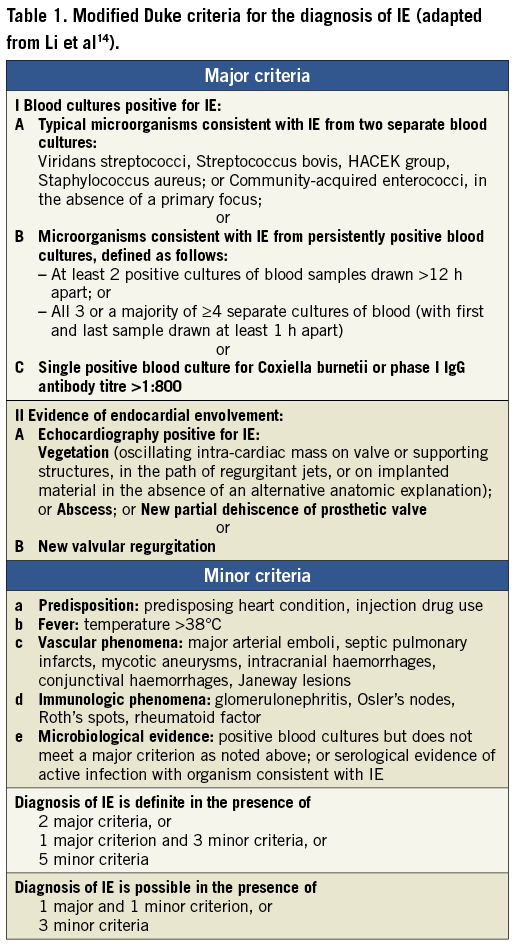
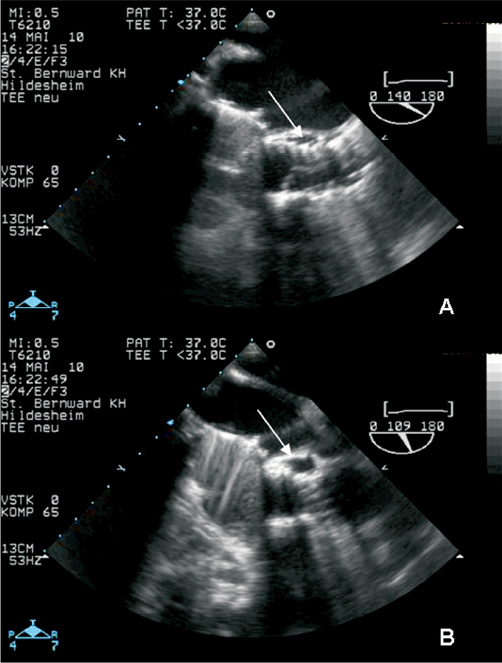
Figure 1. TEE 3-chamber-views of patient 1. The prosthetic mitral valve (SJM) caused extensive shadowing complicating the detection of oscillating intra-cardiac masses. The CoreValve prosthesis seemed not to fit into the aortic annulus and showed a relevant dynamic paravalvular leak (see arrows) enlarging in diastole (B) thereby causing further deformation of the prosthesis. Due to diffuse thickening of the aortic root (A), a large echolucent space between prosthesis and aortic root (arrows), and a discontinuity in the native aortic annulus (B), treating physicians made the diagnosis of an aortic root abscess with mycotic aneurysm.
Antibiotic treatment with rifampin, gentamicin and vancomycin was introduced, but the patient died 18 days after hospital admission due to refractory sepsis and cardiac decompensation. Relatives refused an autopsy. According to the modifed Duke criteria14, two major criteria (IA, IIA, Table 1) and three minor criteria (a, b, c) were present permitting the definite diagnosis of endocarditis. With MRSA, a typical pathogen causing early-onset PVE was detected.
CASE 2
The second patient (an 81-year-old woman with acute congestive heart failure and severe aortic stenosis) was treated with TAVI via transapical approach (23 mm Edwards SAPIEN; Edwards Lifesciences, Irvine, CA, USA) in May of 2009. Preoperative logistic EuroSCORE was 48% due to significant comorbidities (end-stage pulmonary disease after lung tuberculosis, pulmonary hypertension, severely reduced left ventricular function, diabetes). Directly after valve implantation, a moderate paravalvular aortic regurgitation (AR) was detectable.
The perioperative course was complicated by smear-positive reactivation tuberculosis necessitating the transferral to a specialised lung clinic. After ten weeks of chemotherapy consisting of isoniazid, rifampin, pyrazinamide, and ethambutol the patient developed fever >38°C. Sputum smear tests had been repeatedly mycobacterium-negative at that time, but several blood cultures were positive for Enterococcus faecalis. Therefore, the patient was readmitted to our hospital for suspected prosthetic valve endocarditis. TEE showed a still moderate paravalvular AR (Figure 2B) due to paravalvular leak and a new mobile vegetation attached to the prosthetic stent (Figure 2A) verifying a definite diagnosis of early-onset PVE (two major and two minor criteria: IA, IIA, a, b). After intravenous administration of vancomycin and rifampin for six weeks the patient could be discharged with an oral chemotherapy of rifampin and myambutol for tuberculosis. Surveillance TEEs at three weeks after induction of endocarditis-specific therapy (Figure 2C and Figure 2D) as well as at nine months post-discharge demonstrated the persisting paravalvular aortic regurgitation and the absence of vegetation. However, the paravalvular leak seemed to have receded after antibiotic treatment.
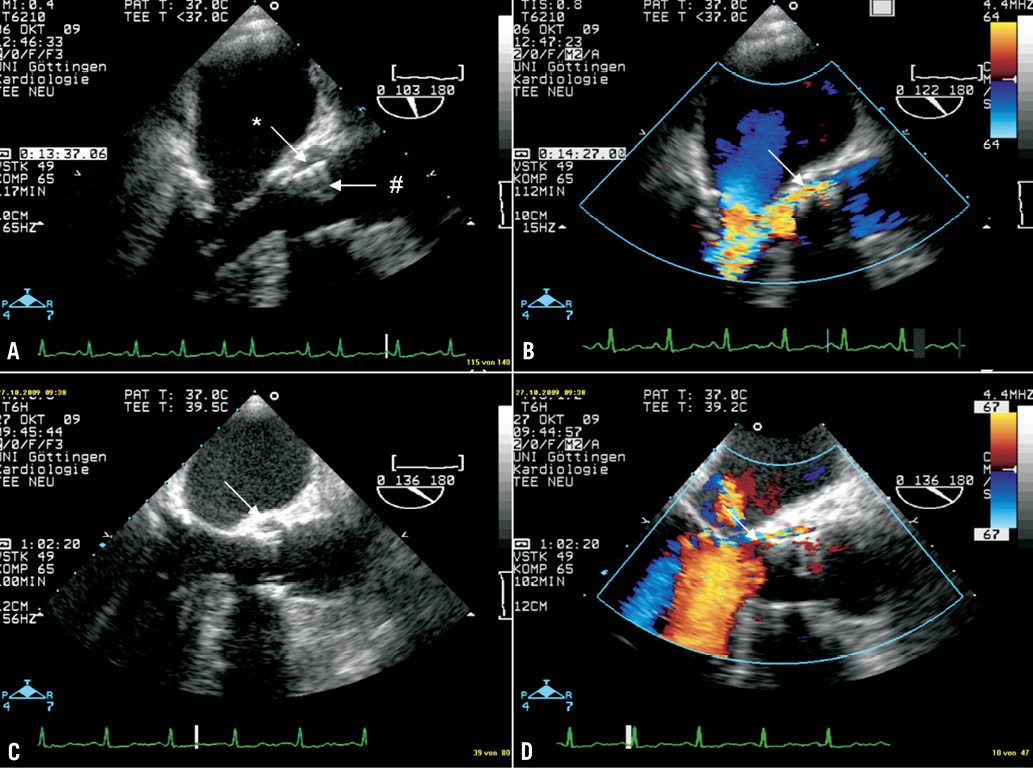
Figure 2. TEE 3-chamber-views of patient 2 (B+D with colour Doppler, C+D surveillance TEE three weeks after A+B). TEE revealed a new mobile vegetation (#) attached to the prosthetic stent (A). Furthermore, a still moderate paravalvular AR (B) due to paravalvular leak (*) was present. Surveillance TEE three weeks later (C and D) demonstrated the persisting paravalvular aortic regurgitation and the absence of vegetation. However, the paravalvular leak seemed to have decreased.
CASE 3
In this case, an Edwards 23 mm prosthesis was implanted in an 80-year-old woman via the transapical approach in March 2010. The preoperative logistic EuroSCORE was 41% due to previous CABG, peripheral artery disease, and chronic lung disease, but the patient experienced no relevant perioperative complications. Echocardiography at discharge demonstrated only trace paravalvular AR.
In January, 2011 she was admitted to a local hospital for recurrent chills. The patient herself had not measured her temperature and during hospital stay the temperature was not >38°C. Several blood cultures were positive for Enterococcus faecalis. TEE showed mild to moderate AR, but no vegetation or abscess could be detected. Since mild AR was seen since the TAVI procedure, the diagnosis of PVE was rejected although extensive imaging revealed no other focus of inflammation. The presence of one major and one minor criterion (IA, a) would already have permitted a “possible diagnosis” of early-onset PVE at that time. Antibiotic treatment with ampicillin for two weeks and then ciprofloxacine for another two weeks was carried out, and the patient was afterwards discharged without laboratory signs of persisting infection.
However, seven weeks later she was readmitted in poor clinical condition after several episodes of collapse. A fever >39°C was measured, and all three blood cultures were again positive for Enterococcus faecalis. Transoesophageal echocardiography now revealed a large oscillating vegetation attached to the prosthetic cusps (Figure 3A, arrow) and a moderate central AR (Figure 3B). In this case, no paravalvular leak was present. According to current guidelines, ampicillin and gentamicin were administered intravenously for six weeks. The vegetation receded under antibiotic therapy (Figure 3C, surveillance TEE after four weeks), but the central at least moderate AR persisted (Figure 3D, surveillance TEE after six weeks). During hospital stay, the patient experienced a minor stroke with temporary paresis of the right arm and dysarthria, but neurologic symptoms had almost completely disappeared at the time of discharge. Cerebral MRI revealed multiple lacunar strokes probably resulting from cardiac embolisation.
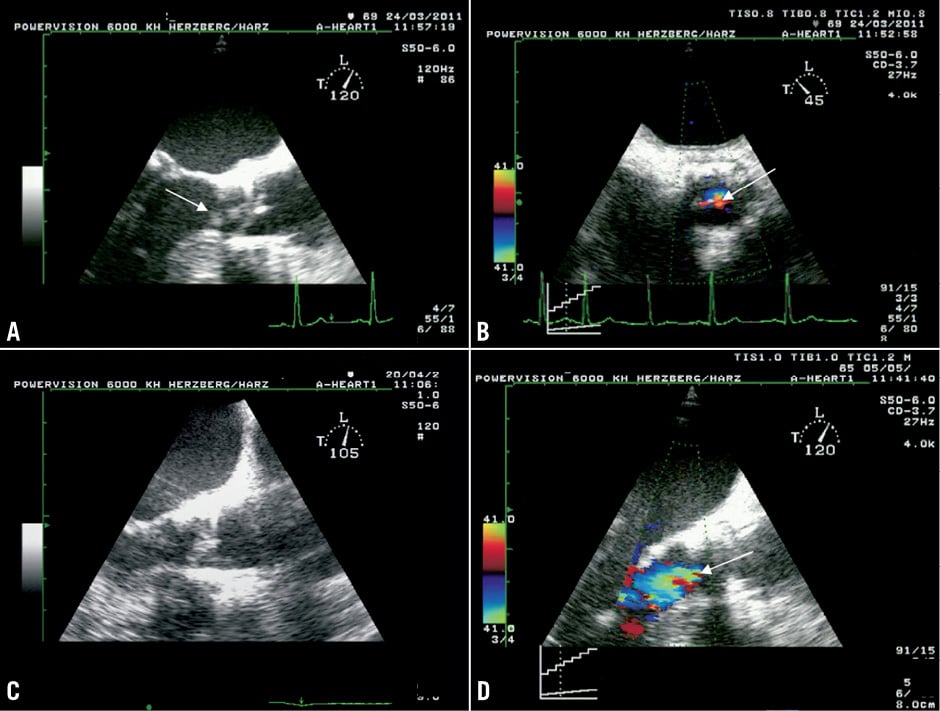
Figure 3. TEE 3-chamber views (A, C, D) and short axis view (B) of patient 3. A large oscillating vegetation attached to the prosthetic cusps (A, arrow) and a moderate central AR (B) were detected. No paravalvular leak was present. The vegetation receded under antibiotic therapy (C, surveillance TEE after 4 weeks), but the central at least moderate AR persisted (D, surveillance TEE after 6 weeks).
At the time of the second hospital admission, two major and three minor criteria (IA, IIA, a, b, c) were present permitting a definite diagnosis of PVE.
CASE 4
An 85-year-old man who suffered from chronic pulmonary disease and pulmonary hypertension (preoperative logistic EuroSCORE 23%) underwent transfemoral TAVI (Edwards 23 mm) in March 2010. The in-hospital stay after the procedure was complicated by the occurrence of acute renal failure with indication for renal replacement therapy, by new-onset third degree atrioventricular block with indication for pacemaker implantation, and by new-onset atrial flutter with indication for ablation. Echocardiography before discharge revealed a moderate paravalvular AR (Figure 4A).
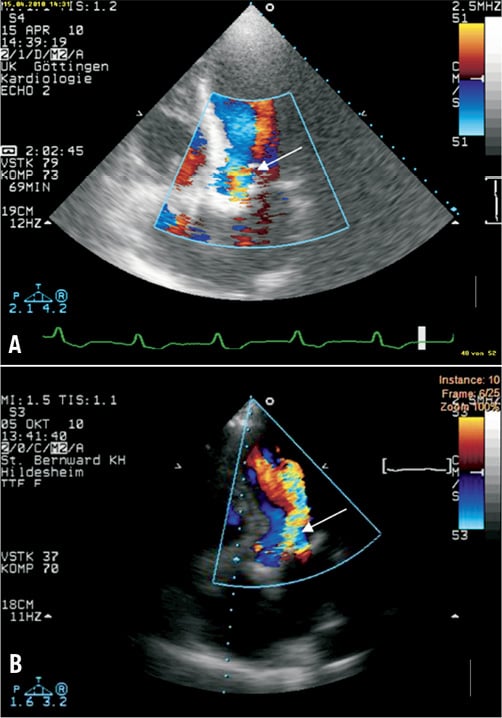
Figure 4. TTE 5-chamber-views of patient 4. Echocardiography directly after the TAVI procedure revealed a moderate paravalvular AR (A). On actual admission transthoracic echocardiography still demonstrated a moderate paravalvular AR without valvular vegetation (B).
In August 2010 the patient was readmitted to the urological department of a local hospital because of fever and urinary tract infection. In urine cultures, Enterococcus faecalis, Escherichia coli, and Candida albicans were detected one after the other. Therefore, three different antibiotic regimens were administered during seven weeks, but recurrent episodes of acute cardiac failure with pleural effusions, recurrent fever under antibiotic therapy and the occurrence of Osler’s nodes on both feet necessitated transferral to the cardiologic department for suspected PVE. Two blood cultures drawn >12 h apart were positive for Escherichia coli (and one of them additionally for Proteus mirabilis), whereas the urine cultures were negative at this time. The patient refused the performance of TEE, but transthoracic echocardiography still demonstrated a moderate paravalvular AR without valvular vegetation (Figure 4B). In spite of parenteral antibiotic treatment, the patient died of septicaemia in December 2010. An autopsy was refused.
According to modified Duke criteria14, one major (IB) and three minor criteria (a, b, d) were present creating a “definite diagnosis” of early-onset PVE with the urinary tract as possible origin of septicaemia.
CASE 5
Transapical TAVI (Edwards 23 mm) was performed in July of 2009 on a 91-year-old woman (preoperative logistic EuroSCORE 25% due to age, gender and pulmonary hypertension). No relevant perioperative complications occurred. At a follow-up visit in August 2010 the patient was in good clinical condition and free of dyspnoea. Echocardiography demonstrated only a trace paravalvular aortic regurgitation, and clinical chemistry showed no signs of inflammation.
The woman was readmitted to our centre in June 2011 due to recurrent fever >38°C and suspected PVE. Three blood cultures were positive for oral streptococci (Streptococcus gordonii). TEE confirmed mild paravalvular aortic regurgitation due to a small paravalvular leak (Figure 5B) that had already been present directly after TAVI (Figure 5A). No vegetation, pseudoaneurysm or abscess was detected.
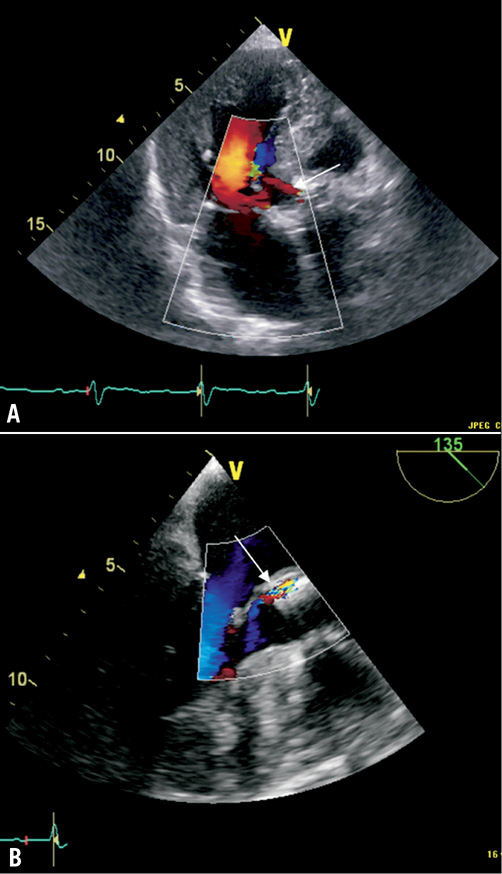
Figure 5. TTE (A) and TEE (B) 3-chamber-views of patient 5. Already directly after the TAVI procedure a mild paravalvular aortic regurgitation due to a small paravalvular leak had been present (A). On actual admission (B), TEE confirmed mild paravalvular aortic regurgitation. No vegetation, mycotic aneurysm or abscess was detected.
Fever associated with a prosthetic valve and positive blood cultures with typical IE causative organisms represent a “possible diagnosis” of in this case late-onset PVE according to modified Duke criteria14 (one major and two minor criteria: IA, a, b). The diagnosis was strengthened by laboratory signs of infection (CRP on admission 80 mg/l, leucocytes 11500/µl). In accordance with current guidelines, the patient was treated with ceftriaxone for four weeks, and could be discharged in good clinical condition afterwards.
Discussion
The anticipated PVE incidence is 0.3-1.2% per patient-year1, and current studies reported markedly lower incidences of early-onset PVE after TAVI than ourselves: 0.6%4, 1.0 %8, 1.1%11, 1.5%7, 2.3%9. Genereux et al recently showed a pooled endocarditis incidence of 0.6% in a meta-analysis of 16 studies (3,519 patients) which applied VARC definitions5. At this present time, the longest follow-up (median, 3.7 years) was carried out by Gurvitch et al6 who described an overall PVE incidence of 1.4 % (n=70 patients, one case of PVE). To our knowledge, only 10 exact case reports providing all relevant information allowing the use of the modified Duke criteria for confirmation of the diagnosis concerning post-TAVI endocarditis have been published18-27.
However, we observed a PVE incidence of 3.4% (95% CI: 0.08 to 6.7) at one year and a person-time incidence rate of 2.99% per patient year in our own patient cohort whose baseline characteristics were comparable to those of preceding publications.
What might be the reasons for the higher than expected incidence of post-TAVI endocarditis in our patients? According to our hypothesis, the diagnosis of PVE might be particularly difficult to make after TAVI, whereas many predisposing conditions might in fact increase the risk of PVE in TAVI-specific elderly patients with significant comorbidities.
Concerning predisposing conditions, the presented cases (Table 2) possess certain similarities. As expected in TAVI procedures, patients were characterised by advanced age (>80 years), high risk for conventional surgery estimated by a high logistic EuroSCORE and numerous comorbidities. Four of five patients with post-TAVI endocarditis exhibited clinically important characteristics possibly related to infectious aetiologies according to previous publications28: two patients suffered from diabetes, one underwent haemodialysis after TAVI, and two were immune-compromised due to reactivation tuberculosis and very old age, respectively. Additionally, one patient showed nasal MRSA colonisation before valve implantation, which might have been the precursor to MRSA bacteraemia and endocarditis.
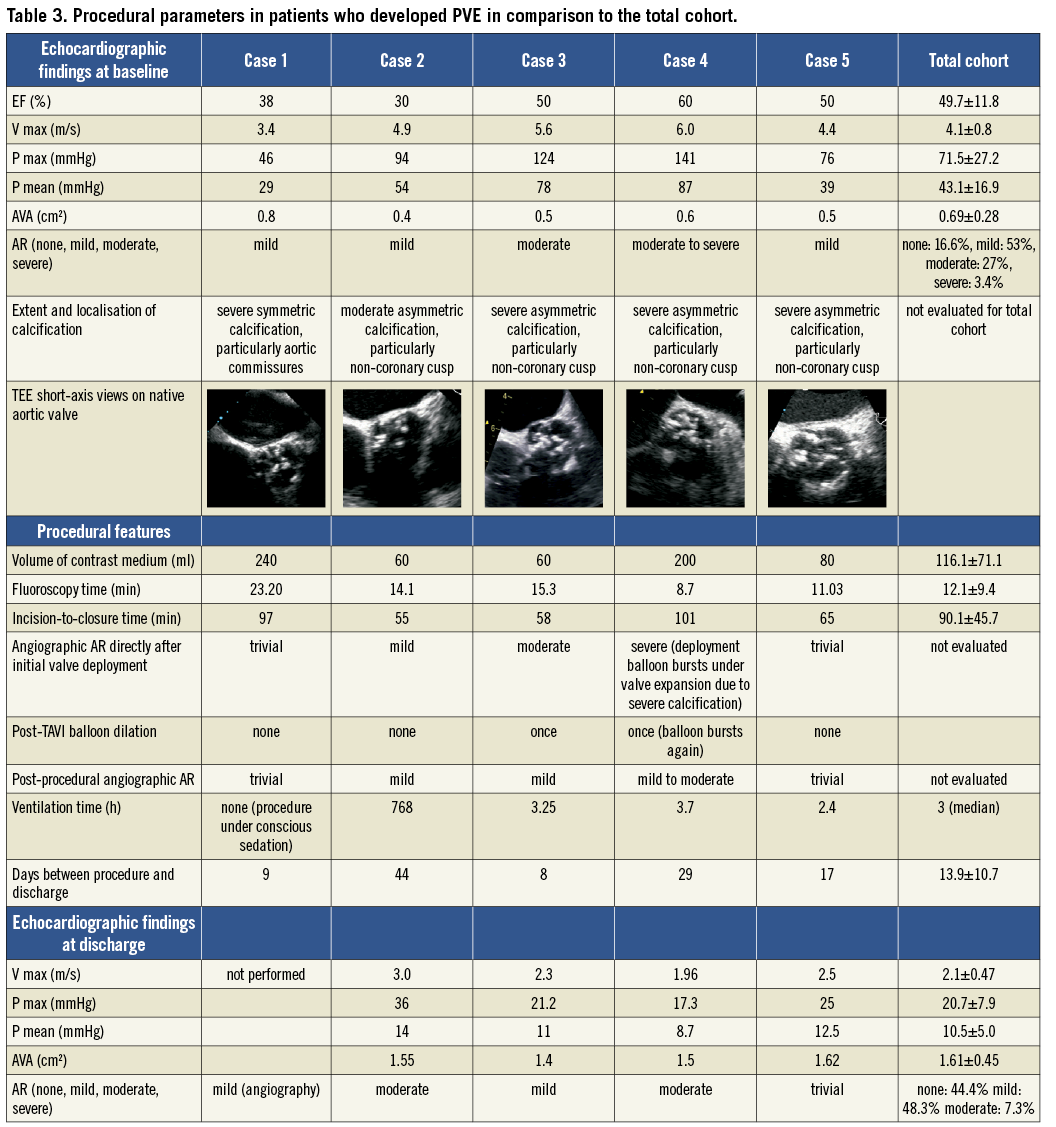
Concerning procedural features, suspected PVE was observed in both valve and access types. The incision-to-closure time, fluoroscopy time and amount of contrast medium used were not higher in patients who developed PVE than in those who did not. Both transapical and transfemoral procedures were always performed by the same operators (one interventional cardiologist and one cardiac surgeon) and the number of nurses and technicians was constant. In cases 3 and 4, post-TAVI balloon dilatation became necessary because of initial moderate (case 3) to severe (case 4) paravalvular AR directly after valve deployment. These two patients were characterised by particularly high transaortic gradients measured by TTE at baseline (Pmean 78/ Pmax 124 mmHg in case 3 and Pmean 87/ Pmax 141 mmHg in case 4). In patient 4, the excessive calcification led to the deployment balloon as well as the post-dilation balloon bursting. Indeed, there was a tendency towards higher pre-procedural transaortic gradients in the PVE cases compared to the total cohort. In four of the five endocarditis patients, the calcification was asymmetric and particularly pronounced on the non-coronary aortic cusp resulting in post-procedural minimal (case 5) to moderate (case 4) paravalvular leakage at the level of the non-coronary cusp. Also, although the incidence of post-procedural moderate AR in the total cohort was only 7.3%, moderate AR at discharge was present in two of the five PVE patients. These findings imply that the functional post-procedural results in the endocarditis group were of a slightly poorer quality than the average result of the total cohort. Echocardiographic findings at baseline and discharge as well as procedural parameters in patients who developed PVE in comparison to the total cohort are summarised in Table 3.
Isolated pathogens were typical for early and late PVE, respectively, with the exception of Escherichia coli (case 4) in which suspected endocarditis followed multiple urinary tract infections as possible portal of entry. In general, E. coli is a rare microorganism in infective endocarditis, and current guidelines report that gram-negative non-HACEK bacteria are found in only 1.8% of endocarditis cases1. However, elderly patients present more frequently than other groups with urinary portal of entry29. A similar case report of post-TAVI PVE following urine infections has already been published18.
Two of our five reported patients (40%) died due to sepsis and pump failure.
In the previously published 10 cases (Table 4), similar baseline characteristics (age mainly >80 years, high logistic EuroSCORE) and multiple predisposing conditions were present. Also, in two patients20,24 a suboptimal device position (too low into the left ventricular outflow tract [LVOT], thereby causing injury and satellite infection of the anterior mitral valve leaflet at the contact point of the ventricular edge of the metal frame) is discussed as a predisposition for the development of infective endocarditis. Of note, the lack of antibiotic prophylaxis perioperatively during dental treatments and colonoscopy is discussed as possible reasons for the development of infective endocarditis in four case reports24-27.
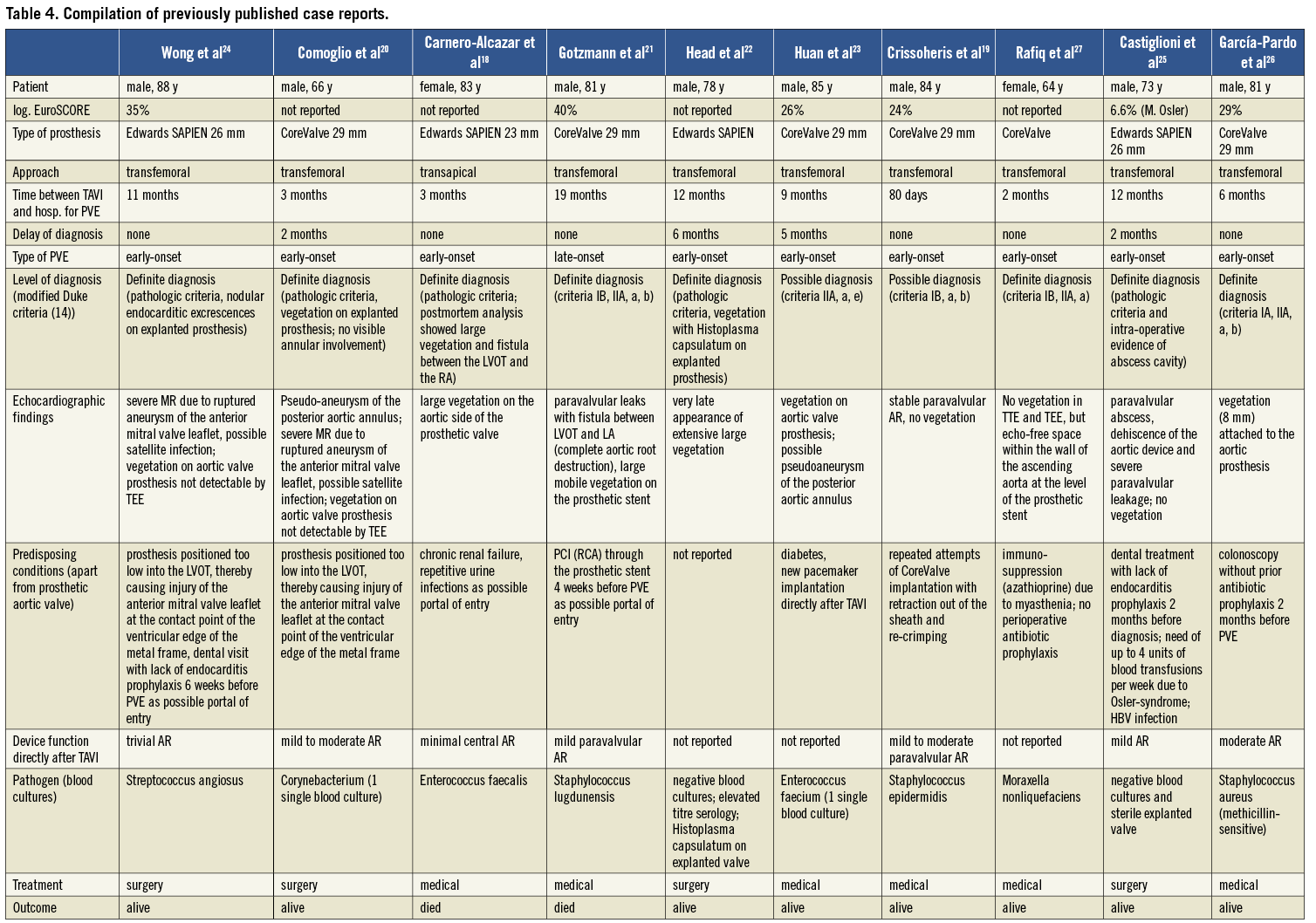
Of the reported 10 patients, two18,21 died of PVE (one case of CoreValve PVE and one of Edwards SAPIEN PVE).
Importantly, a significant delay in PVE diagnosis (2-6 months) was present in four literature case reports20,22,23,25 as well as in one of our own patients (case 3). This was in the one case25 attributable to an atypical clinical presentation (no echocardiography performed until the 1-year routine follow-up), and in four cases attributable to initially inconclusive echocardiography (in combination with microbiological findings not meeting a major criterion according to the Duke-Li criteria14 in the three cases found in the literature). The diagnosis could only be made when massive pathologies like extensive large vegetation, abscesses and fistulas could be detected by TEE. Although all five patients survived, the delay in diagnosis caused significant morbidity (cerebral embolisation, acute renal failure, prolonged hospital stays, open-heart surgery for explantation of infected prosthesis in three cases).
Why is the diagnosis of PVE particularly difficult to make after transcatheter aortic valve implantation?
First, a cornerstone of the diagnosis is a positive echocardiogram including the criteria “vegetation”, “abscess”, “new partial dehiscence of prosthetic valve” and “new valvular regurgitation”14. However, little evidence exists on what an infective endocarditis of a transcatheter aortic valve might look like in TEE imaging. The development of large vegetation is a later event in PVE, whereas the detection of smaller vegetation by TEE is challenging due to the shadowing effect and reflectance of the prosthetic material. Some degree of paravalvular leak, and therefore regurgitation, is common after TAVI because the prosthetic valve sometimes does not fit perfectly into the native valve due to bulky calcifications. Furthermore, early PVE normally involves the junction of the sewing ring and annulus rather than the leaflets alone, leading to valve dehiscence and paravalvular leak30. But, can valve dehiscence arise after TAVI, bearing in mind that the prosthesis is not sutured into the aortic annulus, but “pressed” into the calcifications of the native aortic valve resulting in strong adhesion? There is still little experience about whether a paravalvular leak could progress in the presence of infective endocarditis with the consequence of prosthetic instability. Surveillance TEEs in our own patient 2 suggest that a paravalvular leak can possibly recede under antibiotic therapy. Furthermore, no consensus exists on how an aortic root abscess or a mycotic aneurysm would appear in the presence of a transcatheter aortic valve. In our own patient 1, the treating physicians made the diagnosis of an aortic root abscess with pseudo-aneurysm after a CoreValve implantation (Figure 1). In all previously published cases of CoreValve endocarditis19-21,23 there were also relatively large echolucent spaces detectable between the prosthesis and the posterior aortic wall which might represent abscess cavities. Huan et al23 present images of a surveillance TEE demonstrating a significant increase of this echolucent space (which the authors do not discuss) suggesting that it might indeed represent an abscess formation. In conclusion, the echocardiographic criteria “abscess”, “new partial dehiscence of prosthetic valve” and “new valvular regurgitation” verifying the diagnosis of infective endocarditis are not easily applicable in the context of TAVI. On the other hand, a paravalvular leak is known to be a risk factor for endocarditis after surgical AVR, and the space between transcatheter and native aortic valve might be a particularly suitable nidus for pathogens during transient bacteraemia. Two previously described cases of post-TAVI PVE20,24 and four of our own cases were indeed associated with suboptimal positioning of the prosthesis resulting in mild to moderate paravalvular aortic regurgitation. Incipient vegetation in the free space between transcatheter and native aortic valve, between extensive calcifications and prosthetic material, would be particularly difficult (if at all) to detect by TEE.
Second, IE is a heterogeneous disease with highly variable clinical presentations, and an atypical presentation is common in elderly patients1. This fact might also lead to a significant delay of diagnosis or misdiagnosis resulting in a fatal outcome. For example, two additional patients in our own series died during follow-up due to septic shock of unknown origin shortly after hospital admission. Neither blood cultures were taken nor TEE performed in these patients complicating the confirmation of a diagnosis, but PVE might well have been the focus of septicaemia. Furthermore, in a significant proportion of deaths during follow-up (n=10) no distinct diagnosis was made, but death was attributed to “old age and bad clinical condition”. However, without further diagnostics the patient presented in case 5 could also have ended up in this group, but antibiotic treatment led to a remarkable improvement of her clinical condition.
Taken together, our five cases of post-TAVI PVE and the previously described 10 cases, seven precise case reports on infective endocarditis in CoreValve devices and eight on Edwards SAPIEN PVE have now been published. Four of the 15 patients died of endocarditis (two of seven after CoreValve PVE and two of eight after Edwards SAPIEN PVE) emphasising the seriousness of prosthetic valve endocarditis.
Many of these reported patients met indications for surgery such as locally uncontrolled infection (abscess, pseudoaneurysm, fistula), vegetation >10 mm or heart failure1, but this specific patient population had already been considered too high a risk for open-heart surgery before TAVI. However, surgery was successfully performed in four of the 10 cases found in the literature20,22,24,25 and all of those patients survived. Although little is known about the process of neo-endothelialisation and myofibroblast ingrowth in transcatheter valves, the authors did not report on any serious difficulties in replacing the dysfunctional TAVI devices. Also, three of the four patients who died had clear indications for surgical therapy (abscesses, fistulas, pseudoaneurysms) but were not accepted for open-heart surgery due to prohibitively high risk.
In summary, it is indispensable to be vigilant for PVE after TAVI. Antibiotic prophylaxis should be administered strictly, e.g., before dental treatments. The PVE risk may be even higher in this specific patient population because of advanced age and numerous comorbidities. Furthermore, TAVI patients might be more vulnerable for PVE due to the frequent occurrence of paravalvular leakage as a nidus for infection. Additionally, three of four diagnostic echocardiographic criteria (“abscess”, “new partial dehiscence of prosthetic valve” and “new valvular regurgitation”) are not easily applicable in the context of TAVI, thus complicating the correct diagnosis. The level “definite diagnosis” according to the modified Duke criteria14 is therefore difficult to reach in this setting. Current guidelines do not deal with the problem of infectious endocarditis in transcatheter aortic valves1. In our opinion, positive blood cultures, symptoms compatible with endocarditis and the absence of another focus of infection require the early initiation of an endocarditis-specific antibiotic treatment in TAVI patients, even if echocardiography is inconclusive. In this specific patient cohort, the diagnosis of prosthetic valve endocarditis strongly depends on the evidence of bacteraemia by means of positive blood cultures, which should therefore be drawn repeatedly. Further experience of TEE interpretation in suspected transcatheter valve endocarditis is needed.
Conflict of interest statement
W. Schillinger and R. Seipelt received proctor fees and travel expenses from Edwards Lifesciences. The other authors have no conflicts of interest to declare.

