
Abstract
Aims: The aim of this study was to compare the risk of prosthetic valve endocarditis (PVE) in patients with transcatheter aortic valve replacement (TAVR) or surgical aortic valve replacement (SAVR).
Methods and results: The FinnValve registry included data from 6,463 consecutive patients who underwent TAVR (n=2,130) or SAVR (n=4,333) with a bioprosthesis from 2008 to 2017. PVE was defined according to the modified Duke criteria. In this study, the incidence of PVE was 3.4/1,000 person-years after TAVR, and 2.9/1,000 person-years after SAVR. In competing risk analysis there was no significant difference in the risk of PVE between patients with TAVR and SAVR over an eight-year observational period. Male gender (HR 1.73, 95% CI: 1.04-2.89) and deep sternal wound infection or vascular access-site infection (HR 5.45, 95% CI: 2.24-13.2) were positively associated with PVE, but not type of procedure (HR 1.09, 95% CI: 0.59-2.01) in multivariate analysis. The mortality rate was 37.7% at one month and increased to 52.5% at one year. Surgical treatment was independently associated with decreased in-hospital mortality (HR 0.34, 95% CI: 0.21-0.61).
Conclusions: PVE is rare, and its risk is similar after TAVR and SAVR. ClinicalTrials.gov Identifier: NCT03385915. https://clinicaltrials.gov/ct2/show/NCT03385915
Introduction
Prosthetic valve endocarditis (PVE) has been described as being a causative factor of bioprosthetic valve dysfunction1,2. PVE is rare but is associated with a high mortality rate3,4. The clinical features and outcomes associated with PVE have been well documented in patients undergoing surgical aortic valve replacement (SAVR)5,6, while data on PVE after transcatheter aortic valve replacement (TAVR) are currently very limited.
TAVR has become the dominant treatment strategy for severe aortic stenosis (AS) in patients at high and intermediate risk7,8,9,10. During the past few years, clinical practice has shifted towards also treating lower-risk patients with TAVR11,12. Accordingly, extended knowledge of the durability of TAVR is essential when considering expanding the indication for TAVR to patients with lower risk and those with long life expectancy13. Therefore, the long-term data on bioprosthetic valve dysfunction due to PVE after TAVR are emerging. We sought to investigate 1) the long-term risk of PVE after TAVR in comparison to SAVR, and 2) the clinical outcomes after PVE in the FinnValve registry.
Methods
STUDY DESIGN
The FinnValve registry is a nationwide registry, which includes retrospectively collected data from consecutive and unselected patients who underwent TAVR or SAVR with a bioprosthesis for AS at all five Finnish university hospitals (Helsinki, Kuopio, Oulu, Tampere and Turku) from January 2008 to October 2017. This study was approved by the institutional review boards of each participating centre. The inclusion and exclusion criteria are shown in Supplementary Table 1. The operative risk of the patients was evaluated according to the Society of Thoracic Surgeons (STS-PROM)14 and the EuroSCORE II15 risk scoring methods.
Data were retrospectively collected into a dedicated electronic case report form. Data underwent robust checking of their completeness and quality. Data on date and cause of death were obtained from the national registry Statistics Finland, which is based on death certificates reviewed by local and central authorities. Based on this information, follow-up was considered complete for all patients, except for two patients who were not residing in Finland and for whom follow-up was truncated at hospital discharge.
DEFINITIONS
The definition of PVE was based on the modified Duke criteria16. Cases with definite and possible infective endocarditis (IE) involving the aortic valve prosthesis were considered in this analysis. Any cases considered possible IE were evaluated and finally either included or not in the study on the basis of a consensus of three investigators (N. Moriyama, T. Laakso and M. Laine). Evidence of typical findings of PVE was confirmed by imaging, surgical inspection, or pathological evaluation.
Baseline variables were defined according to the EuroSCORE II criteria15. Frailty was defined according to the geriatric status scale (GSS); herein GSS grades 2-3 were defined as severe17.
OUTCOME MEASURES
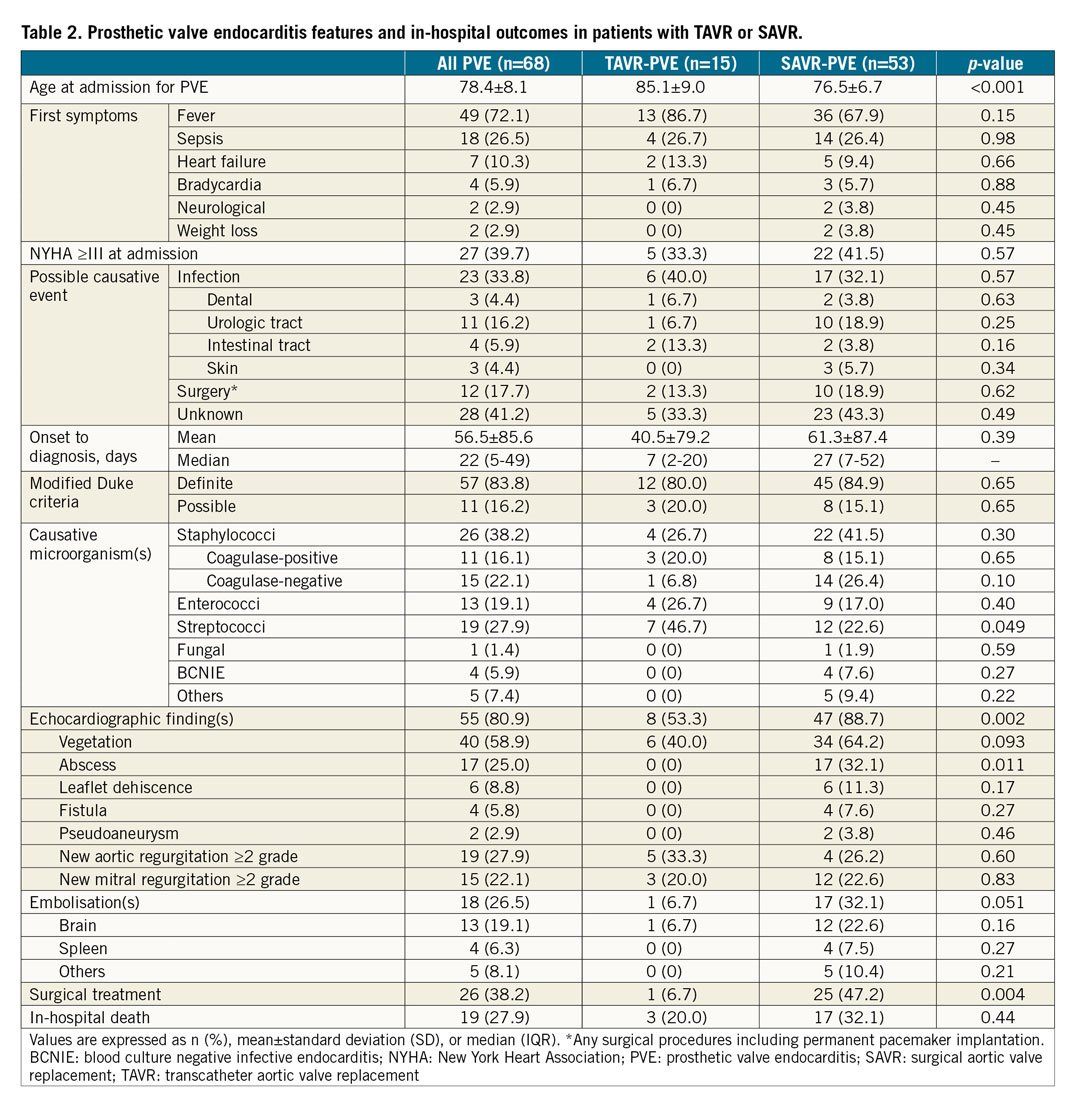
The primary outcome of the study was to define the risk of PVE after TAVR and SAVR. The secondary outcomes were the early adverse events and survival after PVE listed in Table 1 and Table 2.
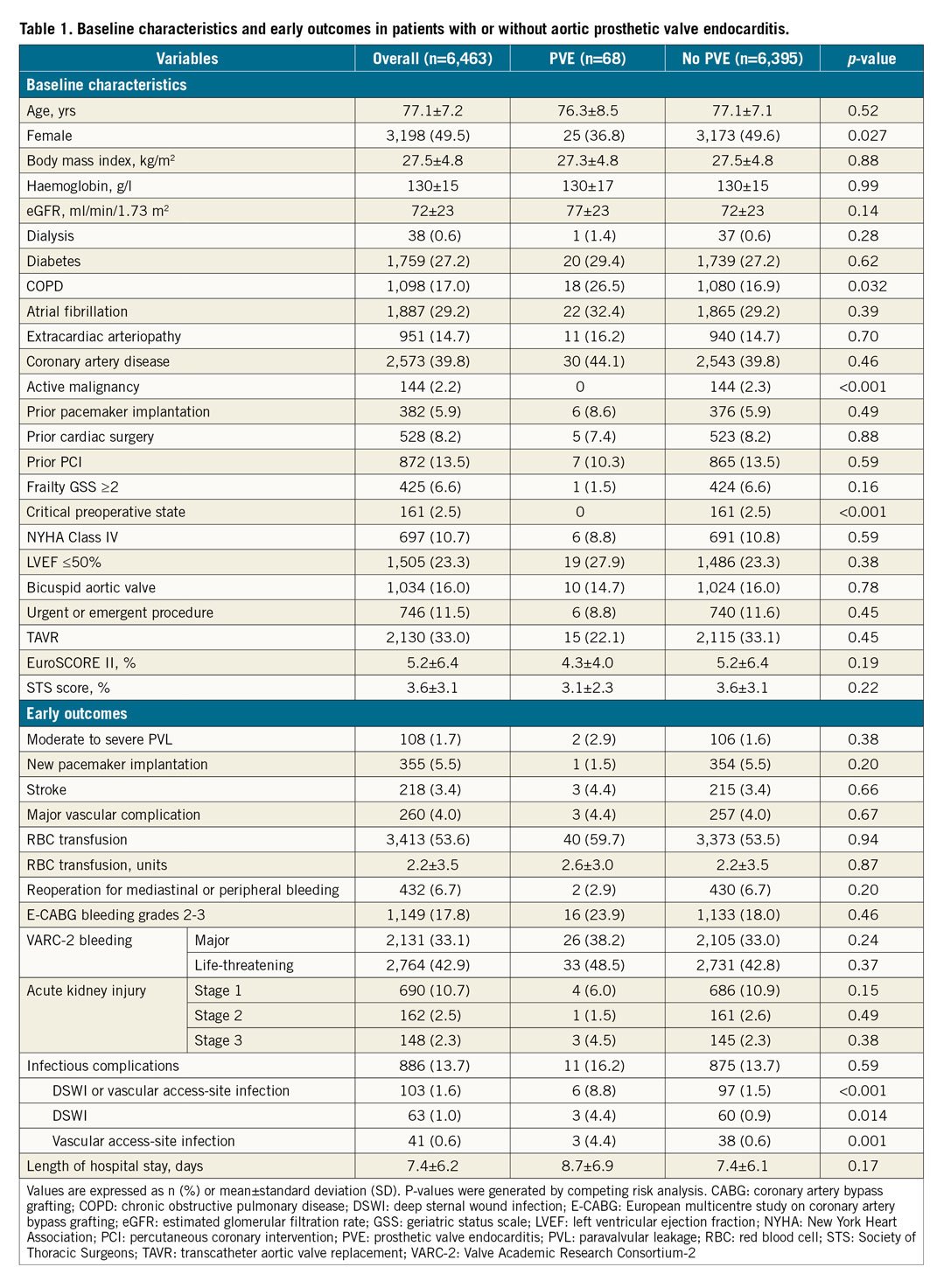
Major bleeding was defined as European multicentre study on coronary artery bypass grafting (E-CABG) bleeding grade 2-318 together with the Valve Academic Research Consortium (VARC)-2 definition of life-threatening and major bleeding. Acute kidney injury (AKI) was defined according to the KDIGO classification criteria19, because it considers a time frame for creatinine changes of seven days, which is usually the average length of hospital stay in patients undergoing SAVR. Other outcomes were defined according to the VARC-2 criteria20. The early outcomes were defined as periprocedural and post-procedural outcomes during the hospital stay for the indexed aortic valve replacement (AVR).
STATISTICAL ANALYSIS
Categorical variables are presented as counts and/or percentages and were compared using the chi-square test. Continuous variables are presented as the mean±standard deviation (SD) or interquartile range (IQR) and were compared using the Student’s t-test or Mann-Whitney U test based on their distributions. Differences in baseline covariates between treatment groups were adjusted using one-to-one propensity score matching analysis with a calliper width of 0.2 of the standard deviation of logit (Supplementary Appendix 1). The risk of PVE was then estimated using competing risk analysis. In competing risk terms, any PVE corresponds to the event of interest. The competing risk was death before PVE. We used cumulative incidence function (CIF) to display the proportion of patients with the event of interest or the competing event as time progressed21. To evaluate the effect of baseline predictors including early outcomes after TAVR or SAVR on the CIF, the Fine and Gray regression model for the sub-distribution hazard was applied22. The following covariates with p<0.20 in univariate analysis were included in the model: age, gender, estimated glomerular filtration rate (eGFR), chronic obstructive pulmonary disease (COPD), active malignancy, critical preoperative state, moderate-to-severe paravalvular regurgitation, reoperation for mediastinal or peripheral bleeding, and deep sternal wound infection (DSWI) or vascular access-site infection. A multivariate analysis was performed to determine the independent predictors of the incidence of PVE. The covariates with p<0.20 in univariate analysis (gender, eGFR, COPD, Frailty GSS 2 to 3, SAVR, DSWI or vascular access-site infection and reoperation for mediastinal or peripheral bleeding [Table 1] and age) were included in the multivariate analysis. Factors at onset of PVE were also analysed to evaluate the risk factors of mortality in patients with PVE using multivariable Cox proportional hazards analysis. Age at the time of PVE was tested as an effect modifier for the relevant covariates. Variables with p<0.2 in univariate analysis were selected for the multivariable analysis. The cumulative mortality and PVE were presented as Kaplan-Meier curves. All hypothesis testing was two-sided with a significance level of 0.05. Statistical analysis was performed using SAS statistical package version 9.2 (SAS Institute Inc., Cary, NC, USA), SPSS, Version 25.0 statistical software (IBM Corp., Armonk, NY, USA) and Stata v. 15.1 statistical software (StataCorp LLC, College Station, TX, USA).
Results
The FinnValve registry includes 6,463 patients who underwent primary TAVR or SAVR with a bioprosthesis for AS: 2,130 (33.0%) patients underwent TAVR and 4,333 (67.0%) underwent SAVR (Supplementary Figure 1). The mean follow-up was 3.5±2.6 years (median 3.0, IQR 1.3-5.2 years, range 0-10.0 years) in the overall cohorts, 3.1±1.7 years in the TAVR cohort, and 4.2±2.6 years in the SAVR cohort.
PROPENSITY SCORE MATCHING MODEL
TAVR and SAVR groups differed in most baseline covariates as shown by standardised differences (Supplementary Table 2). Propensity score matching provided 1,252 pairs with similar characteristics (STS score: TAVR 3.9±2.6% vs. SAVR 4.1±3.7%, p=0.22, EuroSCORE II: TAVR 5.4±5.6% vs. SAVR 5.6±6.6%, p=0.14) as well as standardised differences <0.1 for all covariates. Among the matched pairs, the competing risk analysis showed that the risk of PVE was similar between the study groups (SAVR vs. TAVR, HR 1.67, 95% CI: 0.61-4.59) (Supplementary Figure 2).
OVERALL SERIES
A total of 68 cases of PVE were identified including 15 PVEs in the TAVR and 53 PVEs in the SAVR cohort. The mean time between the indexed AVR and the diagnosis of PVE was 2.1±2.4 years. The overall incidence of PVE was 3.0/1,000 person-years (3.4/1,000 person-years after TAVR, and 2.9/1,000 person-years after SAVR). The main baseline characteristics and early outcomes of patients who experienced PVE are summarised in Table 1. No significant difference in the risk of PVE between TAVR and SAVR was observed over an observational period (Figure 1).
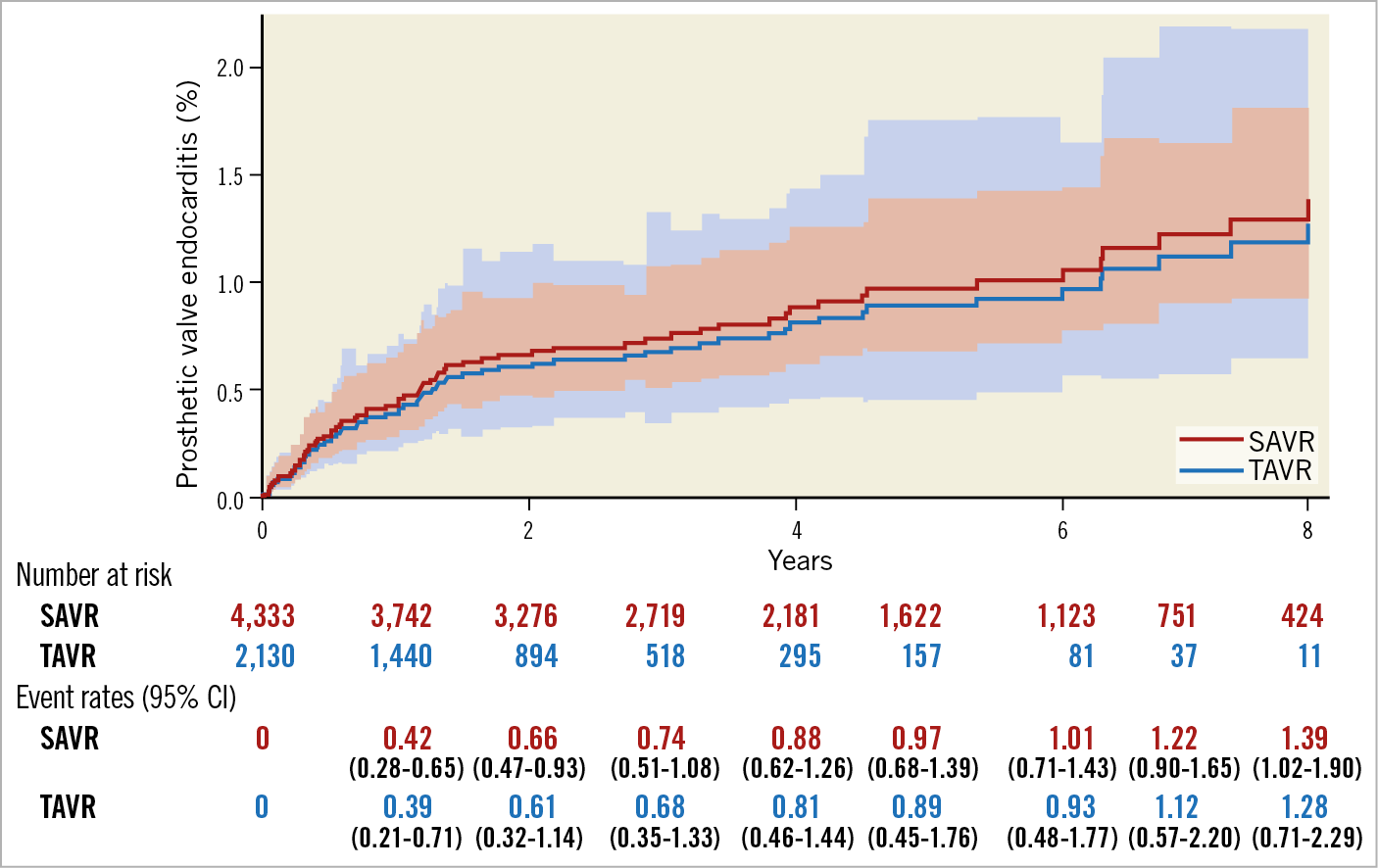
Figure 1. The risk in competing risk analysis with the occurrence of prosthetic valve endocarditis after TAVR and SAVR. There was no significant difference in the risk of PVE between TAVR and SAVR over an eight-year period. CI: confidence interval; SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement
Multivariate analysis showed that male gender (HR 1.73, 95% CI: 1.04-2.89) and DSWI or vascular access-site infection (HR 5.45, 95% CI: 2.24-13.2) were independently associated with PVE (Figure 2).
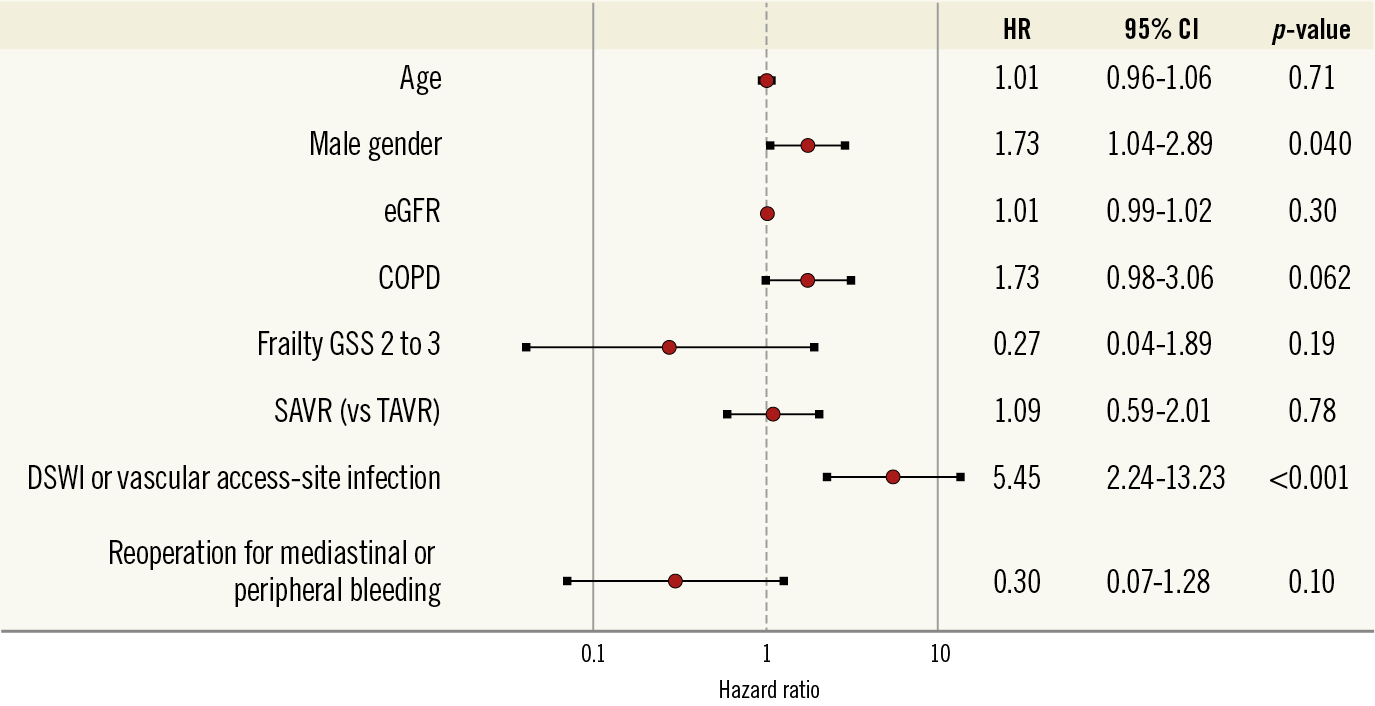
Figure 2. Factors associated with the incidence of prosthetic valve endocarditis following aortic valve replacement with a bioprosthesis. Multivariate analysis including patients’ baseline covariates and early adverse events. CI: confidence interval; COPD: chronic obstructive pulmonary disease; DSWI: deep sternal wound infection; eGFR: estimated glomerular filtration rate; GSS: geriatric status scale; SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement
The main clinical features of PVE are shown in Table 2. Among 68 patients with PVE, staphylococci were the most frequent causal microorganisms (38.2%). Surgical treatment was less frequently performed in the TAVR cohort compared with the SAVR cohort (6.7% vs. 47.2%, p=0.004). In-hospital death occurred in 19 patients (27.9%). The detailed individual data of patients with PVE are reported in Supplementary Table 3. Surgical treatment for PVE was the only independent predictor of in-hospital death (HR 0.34, 95% CI: 0.21-0.61) (Figure 3). The mortality rate after PVE was 37.7% at one month and 52.5% at 12 months (Figure 4).
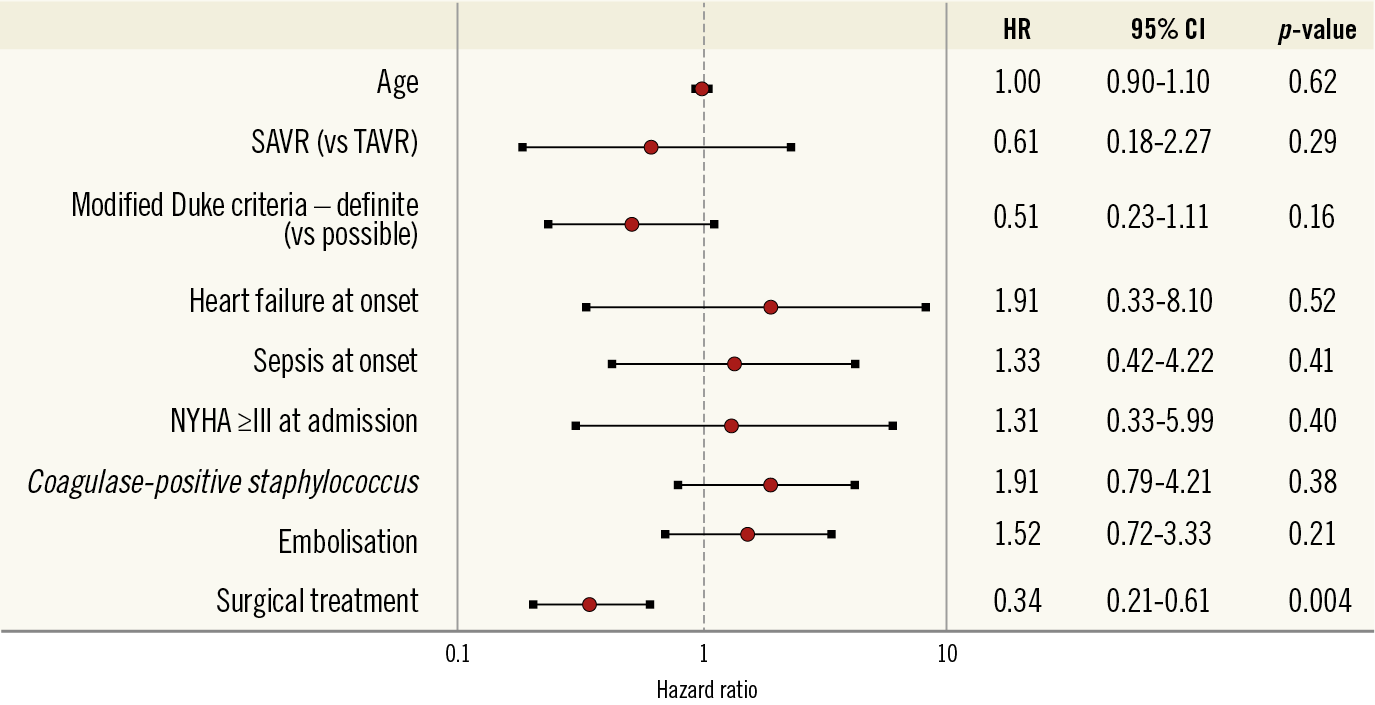
Figure 3. Factors associated with in-hospital mortality following prosthetic valve endocarditis. Multivariate analysis including age at the time of PVE diagnosis and clinical features of patients with PVE. CI: confidence interval; NYHA: New York Heart Association; SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement
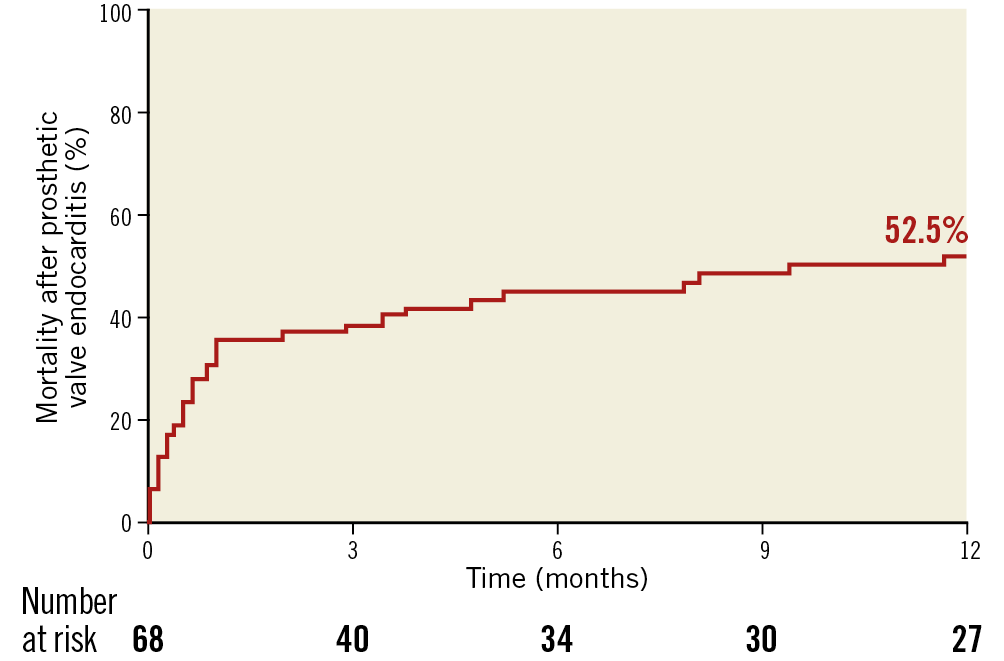
Figure 4. Kaplan-Meier estimate of mortality at 12-month follow-up in patients with prosthetic valve endocarditis. The mortality rate is 37.7% at one month and 52.5% at 12 months.
Discussion
The FinnValve registry showed that there was no difference in the risk of PVE after TAVR and SAVR over time. We also observed that the incidence of PVE was significantly associated with male gender and DSWI or vascular access-site infection following AVR. Furthermore, an excessive rate of mortality was observed in patients who developed PVE.
The incidence of PVE after SAVR is well estimated, ranging from 3 to 12/1,000 person-years6, similar to the overall incidence of 3.0/1,000 person-years observed in this study. With regard to TAVR, the incidence of PVE has been reported to be between 0.3% and 3.4% at one-year follow-up4,23,24,25,26. In the PARTNER trial, PVE at five years occurred in 2.0% of patients with TAVR27. Currently, the data on PVE beyond five years are scarce. In this study, we used a competing risk method to elucidate the risk of PVE as suggested by a consensus statement1. Indeed, in this context the Kaplan-Meier method censors patients who die before the occurrence of PVE; this may lead to an overestimation of the risk of PVE21,28,29. Using the competing risk regression method, we observed that the risk of PVE was similar after TAVR and SAVR.
Our study showed an independent association between the development of PVE and male gender. A meta-analysis suggested that native valve endocarditis occurs more frequently in males30. Østergaard et al reported that PVE is also more common in males31. These reports support a gender difference regarding the risk of PVE. The potential mechanism of less frequent PVE in females could be partially explained by endothelial protection by oestrogen release32. Furthermore, DSWI or vascular access-site infection was significantly associated with an increased risk of PVE. El-Ahdab et al reported that bacteraemia after AVR is highly associated with an increased risk of PVE33. Since surgical and vascular access-site infection are the possible causes of bacteraemia leading to PVE, a strategy of prolonged antibiotic therapy may be indicated in such patients.
PVE is a critical condition, with a risk of in-hospital mortality of 23% to 40%34,35,36. Patients with PVE due to coagulase-positive staphylococcus revealed more severe conditions than those with PVE due to other organisms (Supplementary Table 4). However, surgical treatment was only associated with significantly decreased in-hospital mortality. Nevertheless, the rate of surgical treatment was very low in the TAVR group in the current study. We should acknowledge that only surgical treatment can improve the prognosis of patients with PVE despite the high surgical risk.
Limitations
Our study has several limitations, mainly related to its retrospective nature. Second, the diagnosis of PVE has been well validated by several experienced cardiologists and cardiac surgeons. However, there was no external monitoring committee to verify the accuracy of the data reported by each centre. This may have led to underestimation of the incidence of PVE. Finally, the influence of unknown confounding factors other than those included in the multivariate model for the incidence of PVE cannot be ruled out.
Conclusions
The risk of PVE after TAVR is similar to that following SAVR over time. Patients who develop PVE have a high rate of mortality. These results may have clinical impact on our decision making when we consider expanding the indication of TAVR to low-risk, especially younger populations. Further studies are needed to improve the management of such a critical complication.
|
Impact on daily practice In patients who underwent aortic valve replacement, PVE is very rare. Durability of TAVR in terms of PVE is similar to SAVR with a bioprosthesis over time. Prosthetic valve endocarditis is associated with a high rate of mortality. Further studies are needed to improve the prognosis of patients who have PVE after aortic valve replacement. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

