
Abstract
Infective endocarditis (IE) after transcatheter aortic valve implantation (TAVI) is a new disease entity. The rate of IE after TAVI is similar to that after surgical aortic valve replacement (SAVR), but mortality and prevalence of Enterococcus spp. as causing pathogens are significantly higher. Guidelines on infection prevention measures before TAVI procedures are currently lacking. We performed a structured review of the available data to provide interim recommendations based on guidelines to prevent infections issued by the World Health Organization as well as guidelines by professional societies from Europe and the USA. Such interim recommendations based on expert opinions are probably justified until large randomised trials provide strong evidence for infection control in TAVI, because IE after TAVI is often related to the TAVI procedure itself and the associated mortality rate is high. Antibiotic prophylaxis should be adapted from an intravenous cephalosporin to, e.g., amoxicillin/clavulanic acid, to cover enterococci. In addition, infection control should follow operating room standards as far as is reasonable, even if the evidence for this recommendation is very low. These recommendations are endorsed by the International Society for Cardiovascular Infectious Diseases (ISCVID).
Introduction
Since the first-in-man implantation in 2002, transcatheter aortic valve implantation (TAVI) has rapidly evolved into a standard procedure in the treatment of aortic valve stenosis. Initially, only elderly patients with multiple comorbidities at high risk for surgical aortic valve replacement (SAVR) were treated with TAVI1. Subsequently, TAVI was proven to be at least as effective as SAVR in intermediate- and low-risk patients, thereby increasing the number of patients undergoing TAVI2,3.
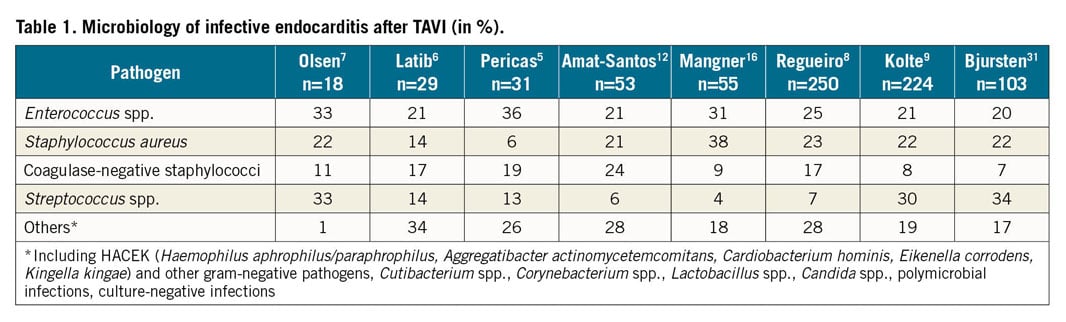
Infective endocarditis (IE) after TAVI was first reported in 20104. The annual incidence varies between 0.2% and 3.4% in retrospective analyses and cohort studies, while a large international registry reported an incidence of 1.1% per person-year (95% CI: 1.1-1.4), which is comparable with rates of IE after SAVR, despite a larger wound surface and a longer surgical procedure in SAVR1,2,5,6,7,8,9,10,11. However, due to the allocation of older patients with multiple comorbidities for TAVI, IE after TAVI is associated with a relevant impact on healthcare costs, morbidity and mortality. In-hospital and one-year mortality rates in IE after TAVI have been reported to be as high as 30-40% and up to 66%, respectively, which exceeds the rate of mortality in IE following SAVR5,8,11,12,13,14. As TAVI is employed using different access routes, relevant differences in the pathogen spectrum were reported when compared with IE after SAVR. In addition, the presence of multiple comorbidities, the higher age and the frequent healthcare contacts of patients with a TAVI prosthesis probably also contribute to the different pathogen spectrum8,15. Compared to native and prosthetic valve IE, where the most common pathogens are Staphylococcus spp. (more than 50%), followed by Streptococcus spp. (30%) and Enterococcus spp. (10%), IE after TAVI is predominantly caused by Enterococcus spp. in 25-30%, of cases, specifically in early infections within the first year of implantation, followed by Staphylococcus and Streptococcus spp. (Table 1),5,6,7,8,9,12,16,17.
The World Health Organization (WHO) and professional societies, including the European Society of Cardiology, have issued guidelines for the prevention of IE after SAVR18,19,20. In contrast, similar published guidelines on infection prevention measures before a TAVI procedure are currently lacking. Cardiac surgery is generally performed in a well-designed operating room (OR) with highly filtrated air, or sophisticated laminar air flow systems, with limited access for healthcare workers, standard surgical gowns and drapes, and well-trained OR nurses – standards that are currently not well defined for TAVI procedures. Therefore, we performed a structured review of the currently available data to provide interim recommendations for TAVI procedures. These recommendations are issued as interim recommendations since large randomised controlled clinical trials are lacking, and observational studies may face serious bias since clinical practices vary widely between hospitals and countries. However, interim recommendations may be even more helpful in situations of uncertainty and harmonising will allow a more structured approach – such as is carried out in registries – to prevent procedure-associated infections including IE.
RISK FACTORS FOR IE AFTER TAVI
Independent associations for the risk of IE after TAVI were male gender, younger age as well as comorbidities including chronic kidney disease and diabetes mellitus8,10. Severe paravalvular aortic regurgitation, redo procedures (including TAVI in a prior TAVI), a low TAVI implantation that interferes with mitral valve closure and generates turbulences, high transvalvular gradients (>50 mmHg) as well as vascular access-site complications were identified as procedure-related risk factors for IE after TAVI7,8,9.
Guidelines for prevention of surgical site infection have been published by the WHO, Centers for Disease Control and Prevention (CDC) and professional medical societies18,21. They include a detailed description of the type of preparation of the surgical site using disinfectants, timing of antimicrobial prophylaxis and recommendations for the environment where SAVR is performed18. The outbreak of Mycobacterium chimaera has recently challenged current cardiac theatre ventilation requirements since the pathogen was transmitted by the airborne route to the newly implanted heart valve22. Among patients undergoing TAVI, Enterococcus spp. belongs to the three most commonly isolated pathogens during IE5,6,7,8,9,12,16,17. The high prevalence of Enterococcus spp. might be explained by differences in the bacterial colonisation between the chest for SAVR and the groin for TAVI, as currently more than 90% of TAVI procedures are performed using the femoral vascular access23,24. However, the high enterococcal prevalence might also be due to higher patient age and the presence of multiple comorbidities, predisposing to frequent healthcare contacts with antibiotic exposure leading to a change of the normal microbiome8,15. The current recommendation for antibiotic prophylaxis for TAVI has been adapted from SAVR routine and includes a cephalosporin focusing on the coverage of the most common pathogen in surgical site infection after cardiac surgery, namely Staphylococcus spp. However, cephalosporins are inherently not active against Enterococcus spp., which belongs to the most commonly isolated pathogens from IE after TAVI25. In addition, many TAVI procedures are performed in catheterisation laboratories, outside of ORs, with clear requirements as for OR standards. Non-surgical staff (nurses and physicians) might not necessarily have been specifically trained for the higher risk for contamination during the procedure, as is well known from SAVR. Periprocedural inguinal skin disinfection might hence follow the requirements for diagnostic cardiac procedures, possibly not being sufficient for a long-term implant. In fact, the level of asepsis is more demanding in implant surgery than, e.g., in abdominal surgery. Alcoholic compounds with chlorhexidine or povidone-iodine are most effective, but these compounds may also be used without alcohol: the exposure time without alcohol increases to up to 10 minutes to be fully effective, a time difficult to follow in a busy catheter laboratory and even more so in cases of emergency. Ventilation does not appear to play a major role, since similar rates of IE are reported, irrespective of whether TAVI was performed in a hybrid OR or in a catheterisation laboratory8,12.
INTERIM INFECTION CONTROL RECOMMENDATIONS FOR THE TAVI PROCEDURE
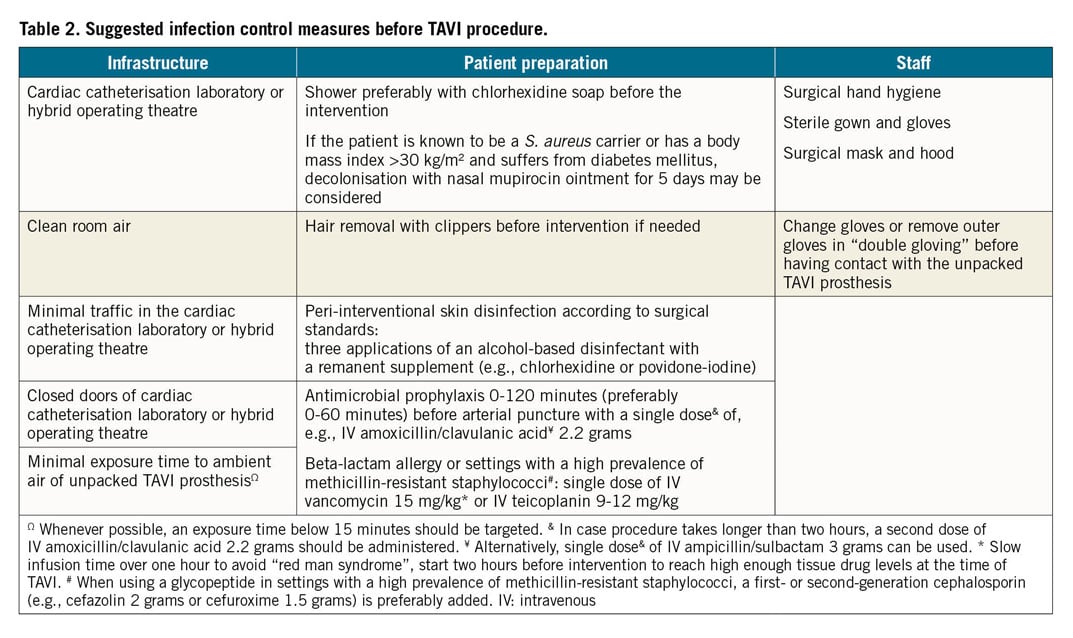
Infection control recommendations adapted from the OR should basically be followed, since IE complications are fatal in up to 40% of cases, with the early period after the procedure carrying the greatest risk for infection. Implementation of these measures should be supervised, e.g., by using the checklist issued by the WHO (Table 2),19.
INFRASTRUCTURE
Interventions are preferably performed in either designated catheterisation laboratories or hybrid ORs, as available, as there is a lack of substantial evidence for the higher standards of an OR8,12. Standards for OR ventilation requirement differ from country to country. Industry standards have also been applied. However, six air changes per hour may be reasonable: today, ISO 14644-1 Cleanroom Standards with ISO class <7 may be useful, but high-efficiency particulate air (HEPA) filters (Type HEPA13) have not been shown to decrease the infection rate and therefore cannot be widely recommended. Traffic in the intervention rooms should be kept to a minimum, doors closed and noise reduced26. The exposure time to ambient air of the unpacked TAVI prosthesis should be as short as possible to avoid contamination; therefore, unpacking is recommended only immediately before the valve is inserted. Whenever possible, an exposure time below 15 minutes should be targeted, as extrapolated from data generated from orthopaedic surgery and animal experiments27. Airborne pathogens can result in contamination of the valve, as observed during open heart surgery with M. chimaera, where the pathogen originated from the ventilator of the computer of the heater-cooler system22.
PATIENTS
An elective TAVI procedure should be postponed if the patient suffers from an active, even remote source of infection, e.g., urosepsis, pneumonia or venous catheter infection, since remote infections are a risk factor for infection of any device to be implanted28. Patient preparation includes whole body showering, preferably with chlorhexidine soap, before the intervention. We suggest informing the patient on the option of decolonisation from Staphylococcus aureus with nasal mupirocin ointment for five days, based on WHO recommendations for SAVR, if the patient is known to be a S. aureus carrier or has a body mass index >30 kg/m² and suffers from diabetes mellitus19,29. Clipping instead of shaving should be used, if hair removal is considered necessary19.
Peri-interventional skin disinfection should be performed according to surgical standards with three applications of an alcohol-based disinfectant with a remanent supplement such as chlorhexidine or povidone-iodine following the manufacturer’s recommendation. Since Enterococcus spp. dominates in IE after TAVI, the commonly recommended antibiotic prophylaxis with an intravenous (IV) cephalosporin (cefazolin or cefuroxime) fails to cover one of the most common pathogens in IE after TAVI; therefore, antimicrobial prophylaxis should be switched to an antibiotic covering enterococci, e.g., IV amoxicillin/clavulanic acid 2.2 grams (single dose) 0-120 minutes (preferably 0-60 minutes) before intervention18. In cases where the TAVI procedure takes longer than two hours, a second dose of IV amoxicillin/clavulanic acid 2.2 grams should be administered25. Intravenous vancomycin (15 mg/kg) and teicoplanin (9-12 mg/kg) are alternatives if the patient is known to be allergic to penicillin or colonised with penicillin-resistant Enterococcus spp. or methicillin-resistant S. aureus. Vancomycin should be infused slowly and preferably two hours prior to intervention to reach high enough tissue drug levels at the time of arterial puncture and to avoid the “red man syndrome”30. When using a glycopeptide in settings with a high prevalence of methicillin-resistant staphylococci, a first- or second-generation cephalosporin (e.g., IV cefazolin 2 grams or cefuroxime 1.5 grams) is preferably added. If vancomycin-resistant enterococci (VRE) are highly prevalent, IV daptomycin (≥10 mg/kg) is one option or teicoplanin in case of VRE VanB.
STAFF
All staff members either having contact with the sterile operation/intervention field or being directly involved in crimping the TAVI prosthesis must perform surgical hand hygiene, and wear sterile gowns and gloves in addition to a surgical hood and mask, recommendations taken from OR guidelines (although they lack strong evidence). All healthcare professionals handling the TAVI prosthesis preferably change to a new pair of sterile gloves before having contact with it, since gloves can rapidly become contaminated in 10-30% of cases either during surgery when preparing the vascular access or while unpacking the TAVI prosthesis.
In addition, every patient should be instructed about personal measures to prevent IE after TAVI, including compliance with strict dental hygiene and with non-specific prevention measures such as no self-medication with antibiotics in case of fever, the renouncement of piercing and tattooing and the timely limitation of intravascular catheters. Furthermore, patients with a TAVI prosthesis should receive an endocarditis prophylaxis document describing prophylactic antibiotic use in different medical interventions such as dental, gastrointestinal, urogenital and infected skin interventions20.
Conclusion
Infective endocarditis after TAVI is a new disease entity which is associated with high rates of morbidity and mortality. The rate of IE after TAVI is similar to that after SAVR, but Enterococcus spp. is three times more common in IE after TAVI. Therefore, antibiotic prophylaxis prior to performing TAVI should be changed from an IV cephalosporin to, e.g., IV amoxicillin/clavulanic acid or an alternative, if the patient is allergic or colonised with resistant pathogens. Prosthetic heart valves, including those used in TAVI, are most likely to become contaminated during the procedure; therefore, prevention should follow OR recommendations as far as reasonable, even if the evidence for this recommendation is very limited. The high mortality in IE after TAVI probably justifies interim recommendations for performing TAVI based on expert opinion until large randomised trials provide strong evidence for infection control in TAVI.
Acknowledgements
P. Moreillon and M. Hannan represent the International Society for Cardiovascular Infectious Diseases (ISCVID) which endorses these expert-based recommendations.
Conflict of interest statement
S. Stortecky reports grants from Abbott Vascular, Boston Scientific, Edwards Lifesciences, and Medtronic, and personal fees from Boston Scientific and Teleflex, outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

