Abstract
There is a growing body of evidence demonstrating the durability of current transcatheter aortic valve implantation (TAVI) devices up to 5 years. However, it is well known that transcatheter aortic valves can degenerate in a manner similar to surgical bioprostheses. In this review we briefly discuss the modes of failure of transcatheter aortic valves and their potential management.
Introduction
Transcatheter aortic valve implantation (TAVI) has been demonstrated to be a viable treatment for inoperable, high-risk and intermediate-risk elderly patients with severe aortic stenosis1. Short- and medium-term outcomes of TAVI have been encouraging1. However, data on long-term transcatheter heart valve (THV) durability and structural integrity remain scarce2,3.
The surgical experience has taught us that the main concern with bioprostheses is their limited durability. In fact, most surgical biological valves degenerate within 10-20 years4. A recent large-scale evaluation of biological (n=24,410) and mechanical (n=14,789) prostheses implanted during surgical aortic valve replacement in patients between the ages of 65 and 80 years found that those receiving bioprostheses had a more than twofold higher risk of reoperation (mainly secondary to bioprosthesis failure), even though the adjusted risk for death was similar between groups4.
Theoretically, THV leaflet trauma can occur at the time of initial valve preparation and compression, balloon dilation, or as a result of suboptimal leaflet coaptation, leaflet folding, or leaflet-frame contact due to asymmetrical frame expansion. Durability of transcatheter valves may therefore theoretically be shorter than that of surgical bioprostheses. To date, the longest available clinical follow-up concerning a substantial number of TAVI patients is limited to five years, at which time excellent valve performance has been demonstrated2,3. However, as with surgical bioprostheses, transcatheter aortic valves are likely to degenerate with time and may eventually require repeat intervention. In this review we briefly discuss the modes of failure of transcatheter aortic valves and their potential management.
Pathogenesis of late transcatheter heart valve degeneration
A variety of modes of failure has been described for surgical bioprostheses, including endocarditis, tissue leaflet degeneration (due to calcification or tissue ingrowth), thrombosis and paravalvular leak (Figure 1)5.
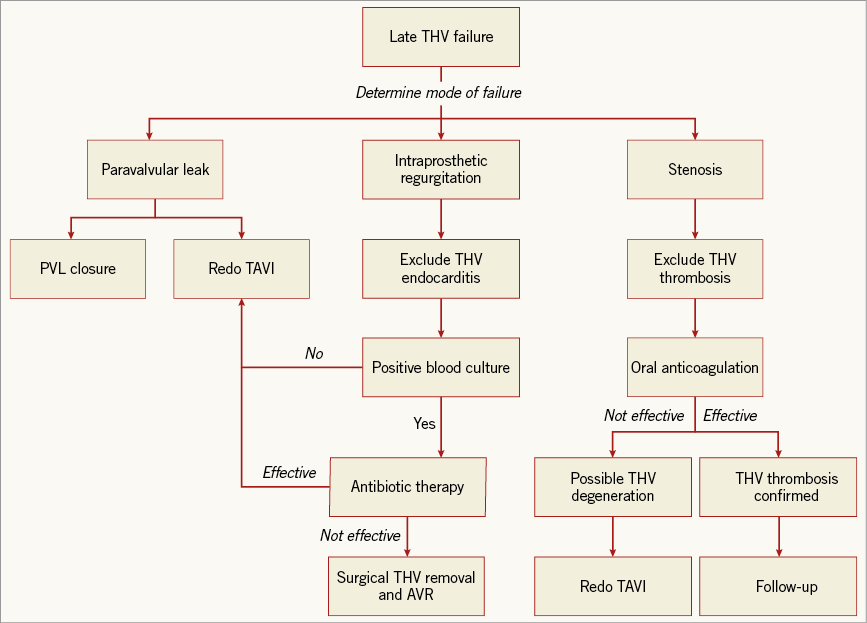
Figure 1. Diagnostic and therapeutic approach to transcatheter heart valve failure. AVR: aortic valve replacement; PVL: paravalvular leak; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve
ENDOCARDITIS
Prosthetic infective endocarditis (IE) after surgical aortic valve replacement is reported to occur in 1-6% of patients and is associated with a high mortality, ranging from 20-80%, despite the ability to diagnose and treat complications5. In patients undergoing TAVI, the reported incidence of prosthetic IE is between 0.5-3.4%5,6. Although it can be argued that the less invasive nature of TAVI could lead to lower rates of early prosthetic IE, it should also be considered that the non-sterile environment of many cardiac catheterisation laboratories and the high-risk profile of TAVI patients could potentially increase the risk of IE after TAVI (Figure 1). Among prosthetic IE cases so far reported in TAVI patients, the predominant causative microorganisms were Staphylococci (31.5%), Enterococci (20%) and Streptococci (14%)6.
DEGENERATION
The most important risk factor for surgical valve degeneration is young age at implantation. Other risk factors include renal failure, hypertension and severe hypercalcaemia7. Bioprosthetic valve degeneration may present as stenosis, which is commonly a result of calcification, or less frequently due to pannus. Alternatively, regurgitation may occur as a consequence of wear and tear but occasionally secondary to calcification7.
Transcatheter heart valves have also been anecdotally demonstrated to degenerate in a manner similar to surgical bioprostheses5. However, it can be argued that the underlying mechanism may be different. In fact, three key differences between TAVI and surgical aortic valve replacement can be identified:
– Aortic root decalcification is not undertaken during TAVI procedures – as a consequence, the combined presence of bulky calcium nodules in the sinuses of Valsalva and the THV may impact on blood flow, causing turbulence in the aortic root with resulting chronic mechanical stresses on the prosthetic valve leaflets, leading to earlier leaflet tissue degeneration. However, this mechanism is purely hypothetical and has never been described in bench and clinical literature.
– Unlike surgical valves, the THV is crimped or loaded into a delivery catheter before being deployed in its anatomical position – leaflet trauma could hypothetically occur during THV preparation and affect long-term durability.
– THVs often have an oval shape (particularly the self-expanding valves) due to constraint by the calcified cusps of the native aortic valve and the left ventricular outflow tract, which usually has a non-circular conformation. These two structures represent the landing/anchoring zones of the THV stent frame. In addition, THVs are generally oversized with respect to the aortic annulus in order to reduce paravalvular leak, frequently producing an incomplete and asymmetrical THV frame shape. However, the impact of THV non-circular conformation on long-term durability is still completely unknown.
THROMBOSIS
Valve thrombosis is emerging as an important issue in TAVI. According to VARC-2 criteria, valve thrombosis is defined as “any thrombus attached to or near an implanted valve that occludes part of the blood flow path, interferes with valve function, or is sufficiently large to warrant treatment”5. This complication is rare, being reported in around 0.5% of TAVI patients5,8. However, what has recently raised the attention of the community is the observation of reduced THV leaflet motion, which is generally subclinical and detected on computed tomography. In the PORTICO IDE trial, Makkar et al demonstrated that this finding was more prevalent among patients who were receiving subtherapeutic or no anticoagulation than among those receiving therapeutic anticoagulation at the time of index computed tomography after TAVI9. With regard to the potential clinical implications of this finding, investigators concluded that the study was small and underpowered for definitive conclusions. In a recent review article, Mylotte and co-workers highlighted several theoretical mechanisms that could potentially increase the risk of THV thrombosis in comparison with surgical bioprostheses5.
– The elderly TAVI population is more likely to have co-existing prothrombotic conditions (e.g., cancer).
– The metallic THV frame could provide a prothrombotic environment.
– Incomplete THV expansion can create leaflet folds and recesses for thrombus formation.
– The native leaflets may overhang short THV devices (i.e., Edwards SAPIEN [Edwards Lifesciences, Irvine, CA, USA], Direct Flow Medical [Direct Flow Medical, Inc., Santa Rosa, CA, USA], Lotus valve [Boston Scientific, Marlborough, MA, USA]), creating areas of diminished blood flow and stagnation.
PARAVALVULAR LEAK
Paravalvular leak is a procedure-related mode of failure which is caused by incomplete apposition of the prosthesis within the aortic annulus due to suboptimal THV deployment. Although this is more frequently an acute complication, the importance of paravalvular regurgitation may only become clinically evident after a period of time, thus requiring further treatment. Next-generation devices are addressing paravalvular leak by external sealing mechanisms and the ability to reposition the prosthesis itself during the deployment process. The issue of paravalvular leak seems likely to be overcome in the near future1.
Management of late transcatheter heart valve degeneration
Understanding the mechanism of late THV failure is of paramount importance as it determines the type of treatment (invasive or medical therapy). Failure of transcatheter prostheses may present as intraprosthetic regurgitation, stenosis, or paravalvular leak (Figure 1).
Intraprosthetic regurgitation occurring late after TAVI is very suggestive of infective endocarditis, particularly when there is no associated valve stenosis5. Targeted antibiotic therapy remains the first line of treatment for THV endocarditis, but THV retrieval and surgical aortic valve replacement should not be discounted in patients with severe intraprosthetic regurgitation5. Alternatively, redo TAVI can be considered a viable alternative when infection has been controlled and blood cultures are negative (data not published) (Figure 1).
Oral anticoagulation is the treatment of choice in patients with clear evidence of THV thrombosis (as well as those with high transvalvular gradient with reduced leaflet motion in the absence of leaflet calcification) (Figure 1). Transvalvular gradients and leaflet mobility often return to normal with this therapeutic regimen8. This observation has raised the question as to whether TAVI should be followed by a certain period of oral anticoagulation in order to prevent THV leaflet thrombosis. Development and implementation of prospective, well-designed and adequately powered studies will shed more light on this important issue.
With the growing worldwide adoption of TAVI and its gradual extension to younger and lower-risk populations, the volume of patients who develop late THV degeneration and require repeat procedures is likely to increase in the future. Although current experience is limited, redo TAVI is feasible and should be considered the standard therapeutic approach for THV degeneration (Figure 1). In a recent international multicentre study of 13,876 patients who underwent TAVI, redo TAVI procedures accounted for only 0.4% of cases. Several aspects argue in their favour. First, redo TAVI appears to be very safe with no increase in the risk of periprocedural complications (in contrast with redo valve surgery which is technically challenging and carries a higher risk of mortality and morbidity than the primary valve procedure)7. Second, valve deployment and sizing are generally facilitated by the presence of the first TAVI prosthesis, which serves as a fluoroscopic marker for the landing zone of the second valve. Conversely, two concerns associated with redo TAVI procedures must be recognised. First, it is unknown whether the presence of two valves could impact on the very long-term durability of the prosthesis. Second, access to the coronary arteries, particularly after implantation of two THVs extending into the ascending aorta (e.g., CoreValve; Medtronic, Minneapolis, MN, USA), needs to be carefully considered.
Conflict of interest statement
M. Barbanti is a consultant for Edwards Lifesciences. C. Tamburino received speaker honoraria from Medtronic Inc., Symetis and St. Jude Medical.

