
The recent successes of transcatheter aortic valve implantation (TAVI) are obvious1,2, but one important question remains: how long does a transcatheter heart valve (THV) last3? The answer to this question has a different meaning according to whether the patient who asks it is 85 (e.g., Adam), 75 (e.g., Bill) or 65 years old (e.g., Chris). Adam has other things to think about and whether the valve lasts 10 or 15 years doesn’t make much difference to him. Bill is rightly more interested in the topic, but feels reassured by the notion that in case of THV failure he will have the opportunity to undergo a new procedure4. Chris was denied TAVI because he is too young: the doctor said that he cannot think of having a TAVI every 10 years for the rest of his life. He really wanted to undergo the procedure and avoid a sternotomy, but the unknowns of THV durability brought him back down to earth. Most physicians would agree that the valve durability-life expectancy ratio is becoming a key driver in the selection of TAVI candidates5.
There are essentially two mechanisms of valve dysfunction, structural and non-structural6. Structural mechanisms include leaflet calcification, leaflet tear, valve seam disruption and stent fracture. Non-structural mechanisms include leaflet thrombosis and endocarditis (which are potentially reversible), paravalvular leakage, and patient-prosthesis mismatch. Non-structural deterioration may result in structural deterioration through mechanisms of leaflet mechanical stress, abnormal flow patterns and inflammation6. A practical classification from the Valve-in-Valve International Data (VIVID) collaboration illustrates structural valve deterioration (SVD) as a continuum, moving from morphological leaflet abnormalities with no significant changes in haemodynamic function (stage 1) to moderate (stage 2) or severe SVD (stage 3) with apparent haemodynamic dysfunction as detected by echocardiography7. In 2017, a consensus document from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) was published, providing standardised definitions of SVD and bioprosthetic valve failure (BVF) for trials of TAVI and surgical aortic valve replacement (SAVR)8. SVD, as an endpoint, encapsulates the perspective of physicians and manufacturers, addressing the crucial question mentioned at the beginning: “how long will this valve last?”. BVF (the composite of valve-related death, re-hospitalisation and severe SVD) encapsulates the perspective of patients, who are more interested in the impact of valve dysfunction on their quality of life and prognosis. According to the EAPCI/ESC/EACTS consensus document, both valve-oriented and patient-oriented endpoints should be considered in the reporting of outcome data after TAVI or SAVR8.
The EAPCI/ESC/EACTS definition has already been embraced by several TAVI studies, enabling meaningful cross-study comparisons (Figure 1)9,10,11,12,13,14,15,16,17. As expected, new definitions bring new criticisms in a constructive spirit18,19,20. A first argument concerns the echocardiographic metrics used to define SVD. The EAPCI/ESC/EACTS definition privileges flow-dependent cut-offs of mean transvalvular gradient, and excludes other flow-independent parameters calculated from left ventricular outflow tract and aortic measurements (e.g., effective orifice area, Doppler velocity index)8. These latter would be useful to refine the diagnosis20, but also impact on simplicity and practicality. A second argument concerns the definition of moderate SVD as “a mean gradient of 20-40 mmHg, or 10-20 mmHg change from baseline, or moderate aortic regurgitation”. Some authors argue that a relative change of 10-20 mmHg from baseline is necessary to make a diagnosis of moderate SVD (e.g., rather than being an alternative to detection of absolute gradients)18,19. The argument behind this observation is the chance to label patient-prosthesis mismatch (e.g., a mean transvalvular gradient of 19 mmHg immediately after SAVR) as moderate SVD if the mean gradient even slightly increases (e.g., +2 from baseline) at an early follow-up after aortic replacement19. Semantically, many would agree that this is not a true deterioration, which may call for a separate terminology and/or separation of patient-prosthesis mismatch from the more inclusive definition of moderate SVD. However, the EAPCI/ESC/EACTS consensus decided to capture and highlight patient-prosthesis mismatch among the reasons for haemodynamic valve dysfunction, because of its established link with the durability of biological valves21. Interestingly, some reactions to the EAPCI/ESC/EACTS definitions came after the observation that moderate SVD is consistently less with TAVI due to higher incidences of patient-prosthesis mismatch after SAVR14,16. This finding becomes apparent when components of the moderate SVD definition are stratified, which is a practice encouraged in reporting (Figure 2). If anything, the EAPCI/ESC/EACTS definition purposely captures absolute and relative differences in haemodynamics between procedures. Indeed, the issue of patient-prosthesis mismatch is not negligible and post-procedural gradients should not be discounted, as they may impact on symptoms, progression to severe SVD, and durability. Finally, it should be noted that the definition of BVF conservatively incorporates severe SVD (e.g., rather than moderate or severe SVD), because the former is less dependent on patient-prosthesis mismatch and more likely to correlate with patients’ symptoms and outcomes8.
In the studies that have so far applied the EAPCI/ESC/EACTS definition of severe SVD, the weighted incidence for THV at five to eight years was 1.3% (95% confidence interval 0.8%-1.9%), while in the studies reporting on BVF the weighted incidence at six to eight years was 4.6% (95% confidence interval 3.0%-6.1%) (Figure 1),9,10,11,12,13,14,15,16,17. Should we expect THVs to deteriorate faster than their surgical counterparts? Theoretically, the answer is yes, because THV leaflets are thinner, subjected to higher stresses and strain, and require crimping6. In addition, THVs have more paravalvular leakage and their duration has been predicated to be eight years shorter on average in tissue-fatigue models22. On the other hand, the answer is no, because THVs have less patient-prosthesis mismatch, larger mean areas post procedure and ample room for improvement in durability with the latest valve iterations as compared with earlier models that have generated most data on durability so far. Moreover, current data show that THVs perform at least similarly to surgical bioprosthetic aortic valves at five to six years. In the two trials reporting on the comparison of TAVI and SAVR using the EAPCI/ESC/EACTS definition, a difference in moderate SVD was noted in favour of TAVI, driven by a decrease both in patients with an absolute mean gradient of 20-40 mmHg and in patients with a relative change of 10-20 mmHg (Figure 2),14,16. However, no difference was noted in severe SVD and – where available – in the incidence of BVF14,16. Comparison of different THVs will probably dominate the next wave of TAVI trials23, generating the prospect of new head-to-head data on durability. The five-year outcomes of the CHOICE trial, currently unpublished, reported significant differences in moderate SVD with first-generation balloon-expandable valves compared with self-expanding valves (5.6% vs 0%, p=0.047), with no differences in severe SVD (presented by Abdel-Wahab et al at EuroPCR 2019, Paris). In moving forward, this is another aspect to follow strictly, because disparities in the way different valve engineering behaves in the long term are plausible.
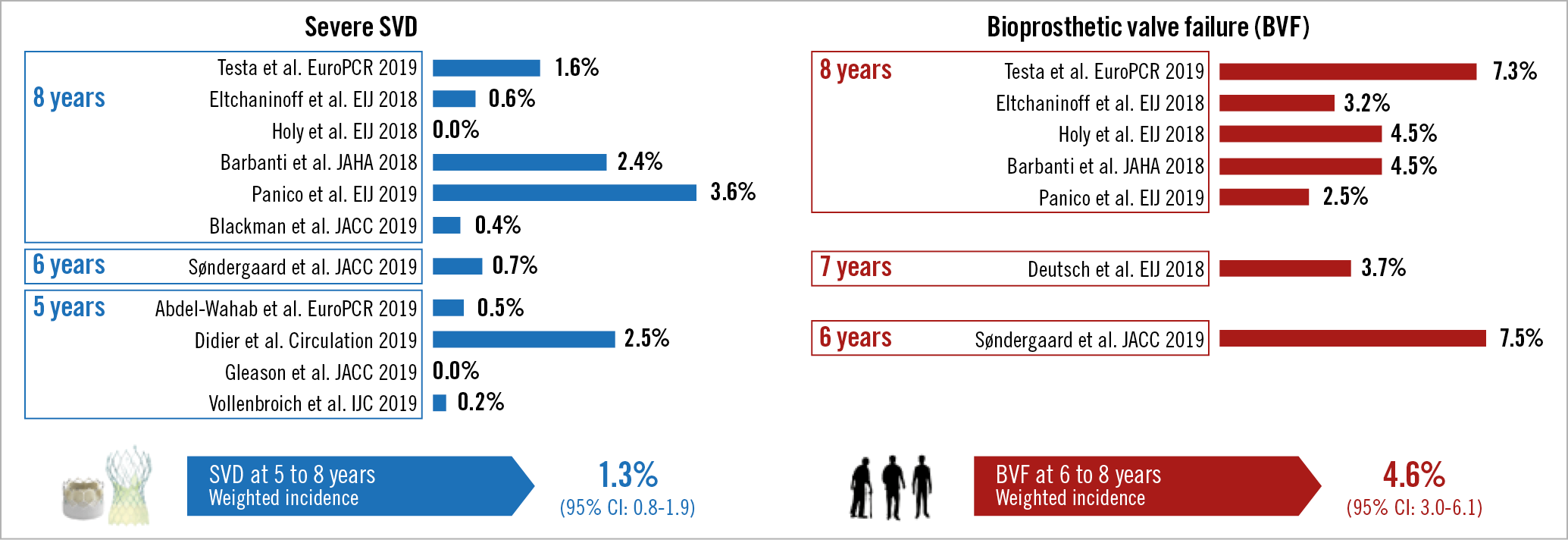
Figure 1. Studies of long-term durability of TAVI using the EAPCI/ESC/EACTS definitions of severe structural valve deterioration (SVD) and bioprosthetic valve failure (BVF).
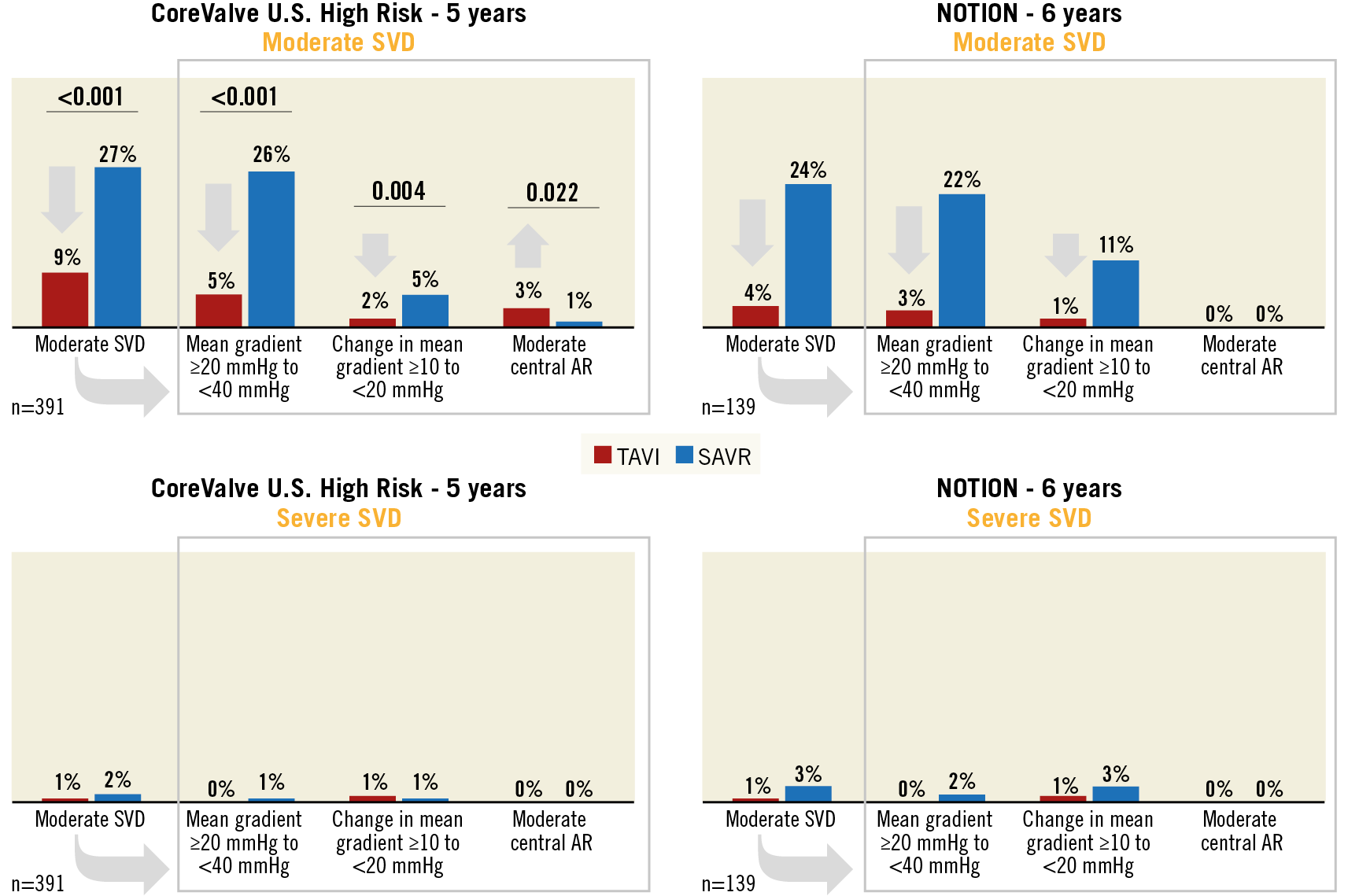
Figure 2. Long-term durability of TAVI versus SAVR in the CoreValve U.S. High Risk and NOTION trials by components of moderate and severe structural valve deterioration (SVD).
The EAPCI/ESC/EACTS rates of SVD and BVF reported so far at eight years are reasonably low and reassuring for the time being. Because what matters most to the abovementioned patients like Bill or Chris is durability at 15 to 20 years, more years of follow-up are needed, and time will tell whether current concerns are well grounded or not. The competing risk of death renders the cohorts of the pivotal trials and registries of TAVI poorly suited for assessing durability, because few patients remain at risk at the time when assessing durability is meaningful. In this respect, assessing long-term durability is more informative when low-risk cohorts are considered8. This, again, requires patience and time for the data to be gathered and appraised. Time will tell, but so far, so good.
Conflict of interest statement
C. Tamburino has received consulting fees from Medtronic. The other authors have no conflicts of interest to declare.

