
More than 15 years have passed since the first transcatheter aortic valve implantation (TAVI)1. Since then, transcatheter technology has evolved significantly and has become a standard therapy for patients considered at high2,3 or intermediate preoperative risk4,5 for surgical aortic valve replacement (SAVR). Notably, the most recent American guidelines for the management of patients with valvular heart disease placed a Class I recommendation for TAVI in prohibitive- and high-risk patients and a Class IIA recommendation for TAVI in intermediate-risk patients6.
As TAVI is increasingly considered in younger patients exhibiting a lower-risk clinical profile, structural valve deterioration (SVD) has become an important concern. Structural valve deterioration refers to acquired intrinsic changes of the bioprosthesis and includes leaflet calcification, tears, stent-frame fracture, and disruption of various components of the bioprosthesis7,8. Non-structural deterioration/degeneration includes intraprosthetic or paravalvular regurgitation, prosthesis malposition, late embolisation, prosthesis-patient mismatch, valve thrombosis, pannus ingrowth and endocarditis7,8.
What do we know about long-term bioprosthetic valve durability?
Many currently available surgical bioprosthetic valves do not have documentation on long-term durability. Although results with newer/contemporary surgical bioprostheses, such as the Trifecta™ (St. Jude Medical, St. Paul, MN, USA), Freedom SOLO™ (Sorin Group [now LivaNova], Milan, Italy) and the sutureless Perceval™ (LivaNova, Milan, Italy) are promising, only midterm follow-up is available at this time. Nonetheless, pooled data from a recent large systematic review show excellent long-term durability for certain bioprostheses after up to 15 years of follow-up9, with reported freedom from reoperation for valve dysfunction at 10, 15 and 20 years of 94%, 82% and 52%, respectively9. It should be noted, though, that freedom from reoperation is a poor marker of bioprosthesis durability; however, for most surgical valves this is the best outcome we have.
We now have excellent documentation with core lab echocardiographic follow-up from randomised trials of both balloon-expandable and self-expanding transcatheter aortic valves (TAVs), showing durability equal to surgical valves at five years10-13. However, we lack rigorous documentation on late durability of TAVs beyond five years14. A recent systematic review of TAV durability reported a pooled incidence rate of SVD of 28 per 10,000 patient years, meaning that 0.6% of patients at two years may experience SVD14. In this analysis, in patients who experienced post-TAVI SVD, the need for valve reintervention for device failure was about 12%14. Although this rate is higher than reported rates of surgical reintervention, it is important to highlight that re-dilating a TAV, implanting a second TAV, or implanting a TAV in a surgical valve is much more likely to be carried out in an elderly patient presenting with a failed TAV or surgical bioprosthetic valve than re-operation in an elderly patient with a failed surgical valve. Hence, the rates of valve reintervention for transcatheter and surgical valves might not be comparable.
Standardising definitions for SVD
Most studies of TAV durability have examined long-term valve performance using Doppler echocardiography. In such studies, SVD is usually defined based on increased mean transprosthetic gradient and less often on the need for valve reintervention for TAV failure14. The lack of standardised definitions has led to uncertainty as to the true incidence of SVD14. In this regard, Capodanno and colleagues8 from the European Association of Percutaneous Cardiovascular Interventions (EAPCI), the European Society of Cardiology (ESC), and the European Association for Cardio-Thoracic Surgery (EACTS) recently published a consensus statement to standardise definitions of SVD and bioprosthetic valve failure (BVF) for assessing long-term durability of transcatheter and surgical bioprosthetic valves. Notably, among others, the authors elegantly describe three different concepts that deserve to be highlighted and are further summarised in Figure 1.
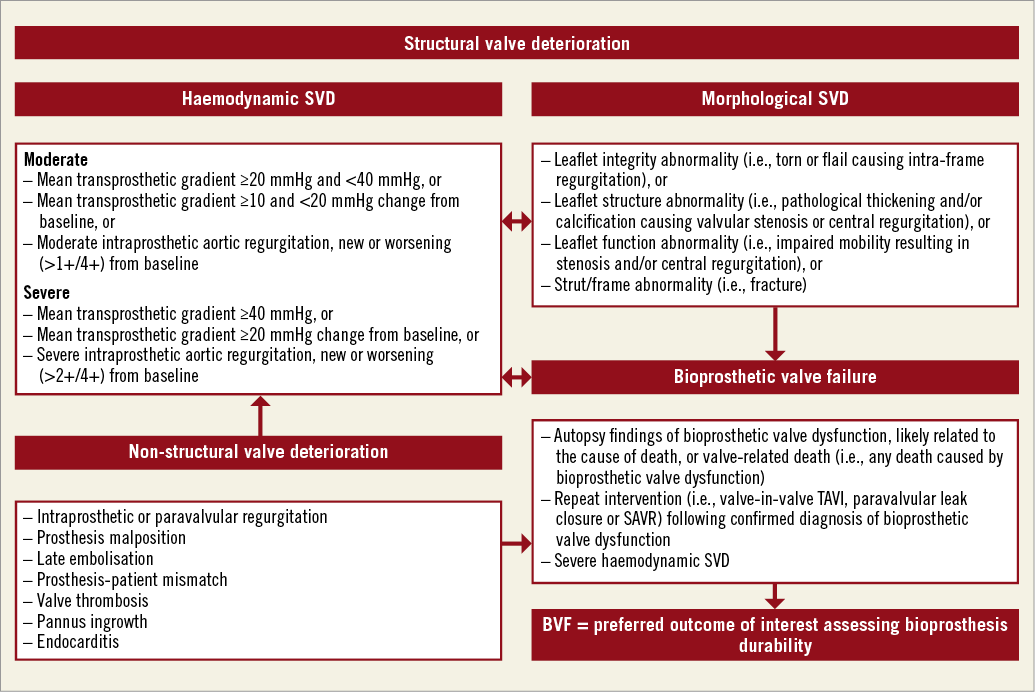
Figure 1. Suggested definitions of structural valve deterioration (SVD) and bioprosthetic valve failure (BVF). Adapted from Capodanno et al8.
HAEMODYNAMIC DYSFUNCTION
This includes patients with increased transprosthetic gradients on echocardiographic follow-up. The Task Force proposed two degrees of haemodynamic SVD. 1) Moderate: mean transprosthetic gradient ≥20 mmHg and <40 mmHg, or change in mean transprosthetic gradient ≥10 and <20 mmHg from baseline, or moderate intraprosthetic aortic regurgitation, new or worsening (>1+/4+ scale) from baseline. 2) Severe: mean transprosthetic gradient ≥40 mmHg, or change in mean transprosthetic gradient ≥20 mmHg from baseline, or severe intraprosthetic aortic regurgitation, new or worsening (>2+/4+ scale) from baseline. The diagnosis is based on permanent haemodynamic changes in bioprosthesis function, regardless of the absence of morphological SVD (“isolated haemodynamic dysfunction”).
MORPHOLOGICAL SVD
This definition includes patients showing bioprosthetic abnormalities affecting leaflet integrity, structure and function, as well as stent/frame. The diagnosis is based on imaging findings, regardless of the need for valve reintervention.
BIOPROSTHETIC VALVE FAILURE
This definition includes patients showing severe SVD and its clinical consequences, i.e., symptomatic patients. Of note, the Task Force recommends BVF as the preferred main outcome of interest in studies assessing the long-term durability of TAVI and SAVR.
Reporting outcomes of interest
Valve-related outcomes refer to the durability of the bioprosthesis, whereas participants per se may be more interested in the probability of having BVF during follow-up (time-dependent). The analysis of the incidence of SVD assumes a constant rate over time. Although this assumption may be true over the first five years of follow-up, it is unlikely to be true over a longer (i.e., 10 years) period of time9,14. In the SAVR arena, it is known that the rates of SVD substantially increase at 10 years, and substantially more again at 15 years9. This pattern of increased hazard of SVD over time may also occur with TAVI, although this has not yet been documented. The Task Force pertinently pointed out the need to recognise competing risks and for informative censoring (i.e., death) while reporting outcomes (i.e., BVF). Hence, when a certain patient is censored, the curve does not take a step down as happens when a patient has the event of interest15.
Personalised medicine and shared decision making
It is conceivable that proper patient selection requires assessment of multiple factors such as baseline comorbidities leading to a certain clinical risk profile, but also the age and potential for longevity of the patient undergoing valve intervention16. From a patient’s perspective, and based on the available data thus far, patients >75 years who place a high value on avoiding open heart surgery are likely to choose TAVI. However, patients in their mid 60s with a potentially longer life expectancy17 may place a particularly high value on avoiding valve reintervention, and thus choose surgery18. Certainly, this scenario must include the discussion around specific surgical valves with demonstrated long-term durability. In this regard, a pragmatic patient-centred approach has been proposed to guide eligibility of choosing TAVI versus SAVR in the form of the “valve durability to life expectancy ratio”17. Hence, a valve durability to life expectancy ratio close to or greater than 1 helps to support a strong recommendation for TAVI over SAVR in this age group, indicating that most patients and physicians would make this choice17. This decision might also be influenced by the implications of what happens when the valve fails. If TAV-in-TAV is available as an option, then this might shift the decision to some extent.
Considering these variabilities in an individual’s values and preferences, a well-informed and shared decision making is of paramount importance for patients being evaluated by the Heart Team. Finally, all the above-mentioned observations underscore the importance of personalised medicine, an approach that goes beyond assessing a single covariate (e.g., SVD or BVF) to determine patient-important outcomes after TAVI.
In summary, our colleagues from Europe are to be congratulated on providing recommendations that establish a landmark for the future reporting of long-term bioprosthetic valve durability. Once again, European colleagues show that they are in the avant-garde – chapeau!
Conflict of interest statement
The authors have no conflicts of interest to declare.

