
Abstract
Aortic bioprostheses are increasingly used both for surgery, as an alternative to mechanical valves, and for transcatheter implantation in patients with severe aortic stenosis. Transcatheter aortic valve implantation (TAVI) has been widely adopted for the treatment of severe symptomatic aortic stenosis in elderly (>75 years) patients who are at risk for surgery and who have favourable transfemoral access. It is already under evaluation in low-risk patients. The aim of this review is to present an update on definitions and to assess the various forms of transcatheter heart valve failure and their management: structural valve deterioration, non-structural deterioration, thrombosis and endocarditis.
Abbreviations
BVD: bioprosthetic valve dysfunction
BVF: bioprosthetic valve failure
BVT: bioprosthetic valve thrombosis
EACTS: European Association for Cardio-Thoracic Surgery
EAPCI: European Association of Percutaneous Cardiovascular Interventions
ESC: European Society of Cardiology
MRI: magnetic resonance imaging
MDCT: multidetector computed tomography
PVE: prosthetic valve endocarditis
SVD: structural valve deterioration
THV: transcatheter heart valve
TAVI: transcatheter aortic valve implantation
TEE: transoesophageal echocardiography
VIVID: Valve-in-Valve International Data
Introduction
Aortic bioprostheses are increasingly used both for surgery, as an alternative to mechanical valves, and for transcatheter implantation. Transcatheter aortic valve implantation (TAVI) has been widely adopted for the treatment of severe symptomatic aortic stenosis in elderly (>75 years) patients who are at risk for surgery and who have favourable transfemoral access1,2.
The transcatheter aortic bioprostheses offer excellent haemodynamics associated with continuously improving short-term results3-5. It is well established that the performance of surgical bioprostheses can be altered by structural valve deterioration (SVD), endocarditis or, more rarely, thrombosis, justifying prophylaxis and long-term antithrombotic therapy.
With a more mature procedure and extension of indications, these well-known surgical limitations require careful evaluation of transcatheter valves. Data on SVD beyond five years are emerging and subclinical thrombosis has gained recent attention6-10. While randomised trials are ongoing in low-risk patients, other trials are investigating the optimal antithrombotic therapy after TAVI.
In this context, the European Association of Percutaneous Cardiovascular Interventions (EAPCI) determined that improved characterisation of long-term TAVI outcomes was timely and proposed standardised definitions of bioprosthetic valve dysfunction11. The aim of this review is to present an update on definitions and to assess the various forms of TAVI failure and their management.
Update on definitions
Transcatheter valves share the biological nature of surgical tissue valves and, consequently, common modes of dysfunction are largely similar between both types of prosthesis. Although some differences in the frequency of occurrence of certain failure modes may exist12, a standardised terminology is essential for accurate assessment of valve function regardless of the implantation mode. This enables an objective evaluation of existing and new transcatheter prostheses as well as their comparative performance versus surgical ones. Therefore, the definitions presented in this chapter should be applicable for both transcatheter and surgically implanted biological tissue valves.
IMPAIRED PERFORMANCE OF BIOPROSTHETIC VALVES: MODES AND DEFINITIONS
The functional performance of a bioprosthetic valve may deteriorate due to various aetiologies. The valve leaflets may degenerate, thrombose, or become infected, and the supporting structures (stent, ring or frame) may fracture, embolise or become dehiscent. In a recent consensus statement, the EAPCI (endorsed by the European Society of Cardiology [ESC] and the European Association for Cardio-Thoracic Surgery [EACTS]) has proposed the term “bioprosthetic valve dysfunction” (BVD) as an overall description of the impaired functional performance of a bioprosthetic valve, which usually manifests as valve stenosis or regurgitation11. This term includes four modes of dysfunction: structural deterioration, non-structural deterioration, thrombosis and endocarditis (Figure 1).
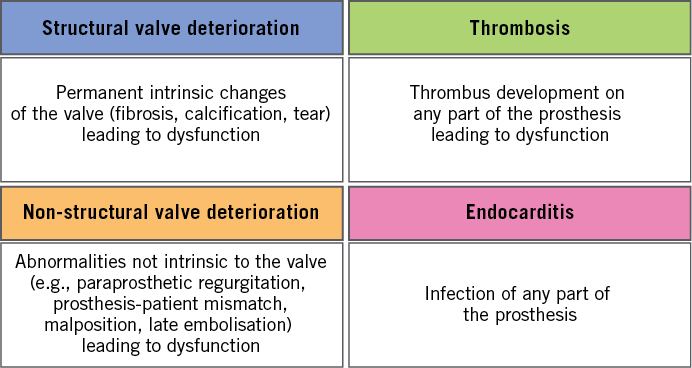
Figure 1. Modes of bioprosthetic valve dysfunction.
Structural valve deterioration (SVD) implies permanent, irreversible, intrinsic changes of the valve. These include, but are not limited to, leaflet fibrosis, calcification, tear, or pannus formation, and are caused by tissue degeneration and/or proliferation involving several pathophysiological processes. SVD may be a pure morphological finding (e.g., during routine echocardiographic evaluation or autopsy) or may result in valve dysfunction (with or without clinical symptoms)11.
Non-structural valve dysfunction includes processes or aetiologies not related to the valve leaflet structure, such as paravalvular regurgitation, patient-prosthesis mismatch, prosthesis malposition and late embolisation11. Some of these processes may occur more commonly after transcatheter compared to surgical replacement (e.g., paravalvular regurgitation), or early after TAVI as a result of technical issues (e.g., malposition).
Thrombosis and endocarditis can cause early valve dysfunction, but could also occur during long-term follow-up after valve implantation. While SVD causes irreversible dysfunction, thrombosis and endocarditis are potentially reversible and should therefore be identified and categorised separately11. However, thrombotic or infectious processes, if they remain undiagnosed or are left untreated, may lead to permanent bioprosthetic valve dysfunction.
STRUCTURAL VALVE DETERIORATION
SVD is probably the most common failure mode of bioprosthetic valves as these are inherently prone to SVD, resulting in limited long-term durability compared to mechanical valves. SVD usually presents as leaflet calcification resulting in stenosis or as leaflet flail or tear resulting in regurgitation or both stenosis and regurgitation combined13. The classic definition of SVD in the surgical literature focused on patient survival without valve reintervention or explant for SVD14. However, recent guidelines and the most recent surgical series have not supported this approach, and SVD was additionally defined by standardised echocardiographic criteria15-18. Importantly, expected valve durability is defined as the median survival time without SVD13.
THE EUROPEAN CONSENSUS DEFINITION
The recently published European consensus statement standardises the definition of SVD for both surgical and transcatheter valves11. It categorises SVD into two types of dysfunction, which may or may not coexist: morphological SVD and haemodynamic SVD (Table 1).
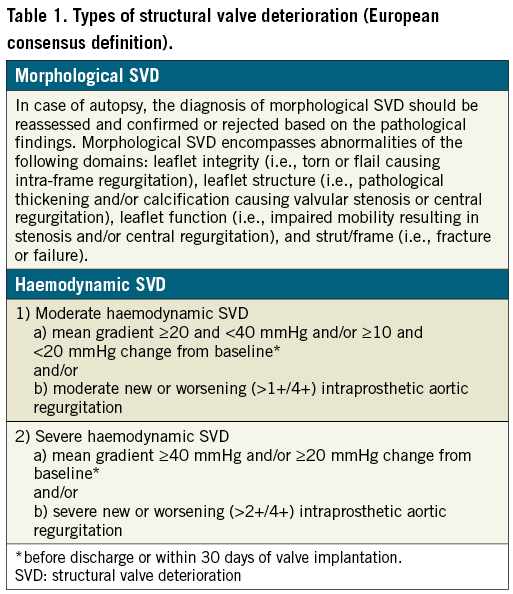
The diagnosis of morphological SVD is based on imaging findings. The diagnosis of haemodynamic SVD depends on the presence of permanent haemodynamic changes in valve function, even without evidence of morphological SVD (so-called isolated haemodynamic SVD), and is further divided into moderate and severe forms depending on strict echocardiographic criteria (Table 1). Importantly, this definition is independent of whether reintervention is performed or not. Reintervention would define valve failure, but is not necessarily a surrogate for SVD.
THE VIVID DEFINITION
Also very recently, the Valve-in-Valve International Data (VIVID) group has published a white paper proposing a standardised definition of SVD13. This definition describes SVD in stages, in an attempt to accommodate the gradual process of bioprosthetic degeneration over time. Stage 1 refers to early morphological changes without haemodynamic affection (this may be equivalent to an “isolated morphological SVD” according to the consensus definition). Stage 2 refers to morphological abnormalities characteristic of SVD associated with moderate dysfunction (stage 2S for moderate stenosis, 2R for moderate regurgitation and 2RS for a combined dysfunction). Although specific echocardiographic criteria for moderate stenosis have not been comprehensively suggested, this definition includes an increase in transvalvular mean gradient of ≥10 mmHg with a concomitant decrease in valve area, emphasising the role of serial imaging studies. Stage 3 SVD is the most advanced form and encompasses severe prosthetic valve stenosis or regurgitation.
BIOPROSTHETIC VALVE FAILURE
The European consensus definition introduces the term bioprosthetic valve failure (BVF), which integrates severe SVD as an aetiological entity with its clinical sequelae11. Importantly, BVF may occur as a result of SVD but also as the consequence of all other previously mentioned causes of BVD (Figure 1). BVF includes any of the following: 1) death probably related to BVD (confirmed by autopsy or by clinical diagnosis of BVD prior to death); 2) repeat intervention (including valve-in-valve TAVI, paravalvular leak closure or surgery); and 3) severe haemodynamic SVD. BVF can be further categorised as definite (autopsy, reintervention, severe haemodynamic SVD) or probable (valve-related death without autopsy), and early (up to 30 days) or late (>30 days) after valve implantation11.
Structural valve deterioration: incidence, mechanisms, management, and comparison with surgery
The predominant mode of BVF is SVD affecting valve durability. SVD is a multifactorial process with two main consequences: calcification and leaflet degradation leading to valve stenosis or leaflet tear with ensuing valve regurgitation.
SVD represents a well-known limitation of surgical bioprostheses and has been widely evaluated in the long term in large series and with various valve models. Durability is usually assessed by survival without reintervention, a clinical definition not taking into account echocardiographic changes and probably underestimating the true rate of SVD. With the exception of some bioprostheses (Mitroflow; Sorin [now LivaNova], Milan, Italy), surgical results are excellent in the long term with survival free of SVD reaching 79% at 15 years with bovine pericardial valves18.
With the growing number of implantations and the expansion of indications, TAVI durability has become a major subject of interest, especially when discussing expansion to lower-risk/younger patients (several ongoing randomised trials: PARTNER 3, EVOLUT R; NOTION 2, UK TAVI Trial). Indeed, TAVI durability might theoretically differ from that of surgical bioprostheses due to valve manufacturing/preparation before delivery with potential leaflet damage after stent crimping and ballooning or non-circular opening of the stent affecting valve haemodynamics19,20.
The absence of homogeneous definitions of SVD and a need to compare transcatheter to surgical bioprostheses led to the previously detailed European consensus on valve failure with new standardised definitions (described above).
Data on long-term durability (i.e., beyond five years) after TAVI are currently very limited, which is inherent to a young technique with only recent worldwide adoption. Indeed, the very low survival rate of the first compassionate and very high-risk patients beyond five years is an important limitation for assessing the long-term durability of transcatheter aortic bioprostheses. Nevertheless, solid data from the large PARTNER randomised trial at five years are reassuring21, with haemodynamic results comparable to surgery and unchanged gradient on annual follow-up. Recently, the five-year incidence of SVD and BVF in TAVI and SAVR patients included in the NOTION trial has been reported at EuroPCR 2018 (unpublished data). SVD was significantly higher after SAVR than after TAVI (26.1% vs. 3.9%, p<0.0001) whereas BVF occurrence was similar (9.5% vs. 8.5%, p=0.89).
Beyond five years, data are very scarce6-8. Eltchaninoff et al6 recently published a series of 378 patients implanted from 2002 to 2012 with balloon-expandable devices using the new definitions of SVD described above. The incidence of SVD was low: 3.3% at eight years with only two severe forms (out of nine). Deutsch et al7 assessed SVD in 300 patients using balloon-expandable and self-expanding devices between 2007 and 2009. At seven years, overall crude cumulative incidence of SVD was higher, at 14.9% (CoreValve® [Medtronic, Minneapolis, MN, USA] 11.8% vs. SAPIEN [Edwards Lifesciences, Irvine, CA, USA] 22.6%; p=0.01). In the third article on long-term durability beyond five years reported by Holy et al8, 152 consecutive patients who had undergone TAVI with the self-expanding CoreValve between 2007 and 2011 at the Heart Center, Bad Segeberg in Germany were evaluated. Echocardiographic follow-up was achieved at 6.3±1.0 years (5.0-8.9 years) and was 88% complete (60 out of 68 survivors beyond five years). SVD was also assessed using the recent European standardised definitions and echocardiograms were analysed by an independent core laboratory. No case showed evidence of SVD. The rare five patients (3.3%) who had undergone redo TAVI or surgery had paravalvular leakage. Finally, Barbanti et al (unpublished data), among a TAVI population of 288 consecutive patients treated with either CoreValve or SAPIEN XT (Edwards Lifesciences) THVs, observed BVF in a total of 11 patients (eight-year cumulative incidence function 4.51%, 95% confidence interval [CI]: 1.95-8.76%). Severe and moderate structural valve dysfunctions were reported in seven patients (eight-year cumulative incidence function 2.39%, 95% CI: 0.77-5.71%) and 13 patients (eight-year cumulative incidence function 5.87%, 95% CI: 3.06-9.96%), respectively. Aortic valve reintervention (redo TAVI) was successfully performed in two patients (0.7%) presenting with symptomatic severe restenosis and intraprosthetic regurgitation subsequent to endocarditis.
Due to the paucity of data and the importance of the topic, SVD is currently being evaluated in large ongoing registries (EAPCI Registry, STOP-AS French registry), and annual clinical and echocardiographic follow-up is now recommended by the European consensus in all patients with either transcatheter or surgical bioprostheses. Recurrence of symptoms, elevated gradient, and new/worsening of central AR require precise diagnosis and management. Indeed, SVD should be differentiated from thrombosis (elevated gradient) or endocarditis (central aortic regurgitation), and transthoracic evaluation should be complemented if necessary by transoesophageal echocardiography and/or MDCT.
Aortic bioprosthetic valve thrombosis
The follow-up of patients receiving surgical bioprosthetic valves has mostly focused on the assessment of long-term SVD, whereas the risk of early bioprosthetic valve thrombosis (BVT) was considered infrequent and irrelevant22,23. The occurrence of aortic BVT six months after TAVI was first described in 200924. Since then, there has been more systematic echocardiographic follow-up and an evolution of transcatheter heart valve (THV) technologies. These advances, together with the highly sensitive nature of four-dimensional computed tomographic (4D CT) imaging to detect subclinical thrombi in both surgically implanted valves and THVs, have generated enormous recent interest in this field.
Two forms of BVT can be schematically distinguished. The first, subclinical, is a recent concept resulting from new 4D CT imaging of leaflet thrombosis9. The second corresponds to clinically overt BVT most often associated with new onset of heart failure symptoms and increase in echo gradients and/or valve insufficiency.
Recently, many studies have evaluated the incidence, prognostic impact, and predictive factors of subclinical aortic BVT mainly after TAVI (Table 2)9,10,25-28. Subclinical BVT, defined by 4D CT as reduced leaflet motion and/or hypoattenuated leaflet thickening, was reported in 6.9% to 40% of cases five days to six months after TAVI29. Subclinical BVT was also reported with surgical aortic bioprostheses but with less frequency, although it has been less investigated9,10. Subclinical BVT is more frequent in patients treated with single or dual antiplatelet therapy than in those on anticoagulant therapy. Furthermore, subclinical BVT seems more frequent with balloon-expandable prostheses than with self-expanding valves9,10. The prognostic impact of subclinical BVT is debated but a small significant increase of transient ischaemic attacks has been reported without increased risk of death, stroke or myocardial infarction9,10. In any case, the recent description of subclinical BVT is of growing interest but requires further investigation. In particular, the ongoing trials comparing TAVI and surgical aortic valve replacement (SAVR) in low-risk patients will systematically address this issue in order to compare the incidence of subclinical BVT in surgical and transcatheter aortic prostheses and to evaluate predictive factors and their prognostic impact further. On the other hand, the recent description of subclinical BVT has led to reconsideration of antithrombotic regimens after TAVI, and many ongoing studies are comparing antiplatelet and anticoagulant therapies in this field.
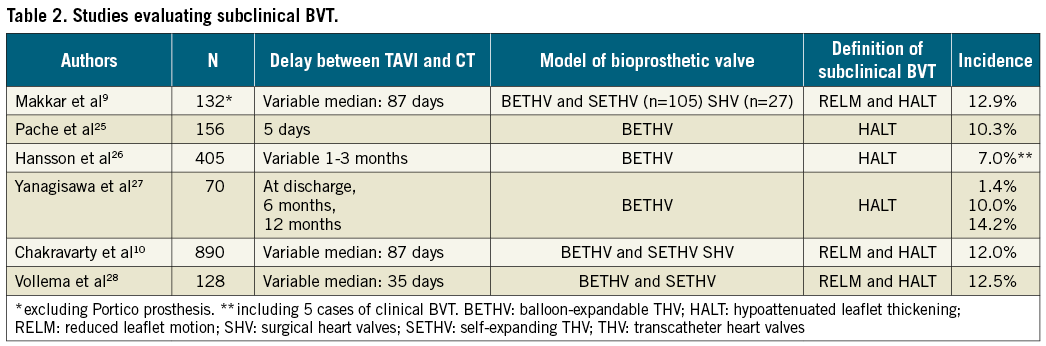
In contrast, clinically overt forms of BVT occur less frequently, with rates ranging between 0.6 and 2.8% of cases30-32. Clinical BVT is mainly diagnosed by the association of heart failure symptoms and elevated gradients three to six months after TAVI, although earlier and later events have also been reported. Clinical BVT is more frequent in patients without anticoagulant therapy, after valve-in-valve procedures and with balloon-expandable prostheses. In most cases, clinical BVT is successfully reversed by anticoagulant therapy, although some patients require fibrinolysis or reintervention. After reversion with anticoagulant therapy, one study reported excellent long-term outcomes with stable echocardiographic findings and no adverse events32.
It is important to distinguish BVT and SVD since clinical and transthoracic echocardiographic presentations are very similar, although BVT classically occurs earlier. Transoesophageal echocardiography (TEE) and/or 4D CT must be performed in order to distinguish BVT and SVD. In some complex cases, or when TEE or CT is not feasible or inconclusive, evaluation of the effect of anticoagulant therapy may be necessary.
Other causes of valve failure (endocarditis and others): incidence, mechanisms and comparison with surgery
The incidence of surgical prosthetic valve endocarditis (PVE) is estimated at 0.3-1.2% per patient-year33. Infective endocarditis has also been shown to be an important causative factor of transcatheter aortic valve failure13. While being relatively rare (1-2% in a recent large multicentre TAVI registry), the prognosis of this complication is detrimental. In a recent multicentre registry that included 250 cases of definite infective endocarditis after TAVI, the in-hospital mortality was 36% and the two-year mortality rate was 66.7%34. Direct comparisons between these rates may be misleading given the complexity of patients undergoing TAVI and heterogeneity in definitions. In the PARTNER (Placement of Aortic Transcatheter Valves) trial (cohort A), prosthetic valve endocarditis occurred at a similar rate in the surgical (1.5%) and transcatheter cohorts (1.0%)21. In the attempt to speculate which approach (TAVI or SAVR) may be more prone to prosthetic endocarditis, on the one hand it can be argued that the less invasive nature of TAVI could be associated with lower rates of early endocarditis, but on the other hand the non-sterile environment of many cardiac catheterisation laboratories and the high-risk profile of TAVI patients could potentially increase the risk of endocarditis.
Mylotte and colleagues12 identified a total of 26 publications, describing 34 cases of transcatheter aortic valve PVE. There were 29 (85%) cases of definite and five (15%) cases of possible endocarditis according to the modified Duke criteria. The median time to infective endocarditis diagnosis was six months, with early (<60 days), intermediate (60 days to 12 months), and late (>12 months) endocarditis classified in 18%, 62%, and 20% of patients, respectively. Enterococcus species, coagulase-negative staphylococci, staphylococcus aureus, streptococcus species, and histoplasma capsulatum were the pathogens involved12.
More recently, Regueiro et al, among a cohort of 250 TAVI patients with a diagnosis of infective endocarditis, found that enterococci species (24.6%; 95% CI: 19.1-30.1%) and staphylococcus aureus (23.3%; 95% CI: 17.9-28.7%) were the most frequently isolated microorganisms34. In addition, the authors showed that younger age, male sex, history of diabetes mellitus, and moderate to severe residual aortic regurgitation were significantly associated with an increased risk of infective endocarditis.
Treatment of transcatheter aortic valve failure
Patients who present severe symptomatic transcatheter aortic valve failure will require reintervention. In this high-risk population, redo TAVI is obviously the preferred option. However, in rare cases, surgery should be discussed when redo TAVI is not feasible (i.e., endocarditis, paravalvular leak…). To date, only a few anecdotal reports and two multicentre international studies have demonstrated the safety and efficacy of redo TAVI to treat post-procedural and late transcatheter valve failure35-38.
Today, redo TAVI procedures are quite rare, accounting for 0.4% of global TAVIs37. However, the volume is expected to grow in the coming years. In the study by Barbanti et al37, the indication for redo TAVI was moderate-severe PVL in 25 patients and valve degeneration (new valve stenosis n=9, intravalvular regurgitation n=13, or combined n=3) in the remaining 25 patients. The mean interval between the index TAVI and redo TAVI was approximately two years. This interval was significantly lower in patients undergoing redo TAVI for PVL (15 months), as compared to patients experiencing SVD (39 months). Redo TAVI procedures were quite safe with only one case (2.0%) of coronary occlusion successfully treated with rescue PCI, and no cases of aortic annulus rupture. After redo TAVI all patients left the hospital alive. During hospitalisation, one patient (2.0%) had a non-disabling stroke, and another patient (2.0%) had a life-threatening bleeding, whereas new permanent pacemaker implantation was required in three out of 35 (8.6%). At a median follow-up of 1,589 (range: 31 to 3,775) and 586 (range: 8 to 2,460) days after index and redo TAVI, respectively, survival was 85.1%. After redo TAVI, valve performance compared favourably with other recent TAVI series. Moderately elevated intraprosthetic gradients (mean gradients ≥20 mmHg) were reported in five patients (10%) presenting with stenosis of the first transcatheter valve, even though in one of these patient-prosthesis mismatch was reported immediately after the first TAVI. In this multicentre analysis, the selection of the prosthesis type and size to perform redo TAVI varied significantly across centres, suggesting that this procedure is still far from being well standardised. From a technical standpoint, the following particular considerations should be given when selecting the prosthesis to treat a degenerated transcatheter aortic valve: 1) guarantee a proper anchoring and sealing of the second transcatheter valve, 2) reduce the risk of prosthesis mismatch, and 3) maintain the free access to the coronary ostia.
Assessment of THV performance and recommendations for follow-up
ASSESSMENT OF THV PERFORMANCE
The previously described failure modes of bioprosthetic valves (including transcatheter ones) may occur early after valve implantation or later on during the patient’s lifespan. Consequently, assessment of THV structure and function should be an integral part of patient care and should rely on both clinical assessment and specific imaging studies.
CLINICAL AND LABORATORY ASSESSMENT
Patients should be clinically evaluated for symptoms and signs of congestive heart failure, embolic complications and systemic infection. In addition to the standard laboratory tests performed in cases with suspected THV failure, brain-type natriuretic peptides may have an additional diagnostic value, particularly if serial measurements are available31,39.
ECHOCARDIOGRAPHY
Transthoracic echocardiography is the main imaging modality for assessment of THV function. It has the ability to characterise valve haemodynamics (transvalvular gradients, effective orifice area and valve regurgitation) adequately as well as morphological changes of the implanted prosthesis (e.g., calcification, thrombosis, vegetations, paravalvular leakage). Periodic echocardiographic surveillance is currently the reference standard for detection of BVD, and stenosis or regurgitation of the THV should be reported using validated quantitative or semiquantitative methods40. Transoesophageal echocardiography can improve morphological visualisation of the valve prosthesis if transthoracic images are suboptimal, and the additional role of three-dimensional echocardiography in this setting is evolving11.
COMPUTED TOMOGRAPHY
Multidetector computed tomography (MDCT) provides less functional but more structural information on implanted THVs. In particular, it is more sensitive than echocardiography in detecting leaflet thrombosis at early stages of the process before overt valve dysfunction25, and has the ability to characterise precisely stent frame position, expansion, and eccentricity, as well as its relationship to the surrounding structures. The latter may be of particular importance if a redo TAVI procedure is planned, e.g., to assess the risk of coronary obstruction if a second prosthesis is implanted. MDCT can also be useful for the differentiation between leaflet thrombosis and pannus formation41, which is of particular importance since treatment strategies for both entities are different (medical treatment by anticoagulation versus reintervention). Importantly, MDCT cannot determine valve gradients and is therefore of limited utility for the diagnosis of SVD11.
CARDIAC MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) has the ability to combine both structural and functional information, but is not always available, and experience in the assessment of BVD remains limited. In addition to its superiority in assessing left and right ventricular cardiac dimensions and function, it has the ability to quantify prosthetic valve regurgitation precisely42,43. Cardiac MRI can be particularly useful in patients with unclear/undetermined severity of prosthetic valve regurgitation by echocardiography, especially when it is of paravalvular origin44.
FOLLOW-UP INTERVALS
For the longitudinal assessment of THV performance, baseline post-procedural studies are essential to have a reliable reference for comparison of new valve findings during follow-up. The standard method remains high-quality echocardiography. Echocardiography is the principal imaging modality for the detection of all forms of BVD, particularly SVD, and the best and most accessible way to detect serial changes in valve function. According to current guideline recommendations, after both transcatheter and surgical valve replacement, echocardiography should be performed before discharge or within 30 days after valve implantation (baseline imaging), at one year after valve implantation and annually thereafter, with additional assessments as well as integration of other imaging modalities (such as MDCT and cardiac MRI) when necessary and as previously described11. Previous recommendations delaying serial valve assessment up to five years after implantation are clearly outdated and are not recommended. Although long-term follow-up beyond five years remains challenging for the elderly population currently treated with TAVI13, assessment of THV durability is crucial and should be integrated in the follow-up schedule of all treated patients.
Conclusion and perspectives
Alain Cribier’s dream of implanting a valve as a stent-like procedure without resorting to heavy surgery in patients with severe symptomatic aortic stenosis has come true. The results even surpass the initial expectations, the procedure offering excellent haemodynamics without the invasiveness surrounding surgery. Valve failure is a well-known limitation of bioprosthetic valves and current data do not demonstrate any alarm in comparison to surgical valves. Standardised definitions, careful follow-up of all patients with bioprostheses and long-term valve failure assessment are warranted and will provide more information on both understanding and optimal management of various forms of valve failure.
Acknowledgement
The authors are grateful to Nikki Sabourin-Gibbs, Rouen University Hospital, for her help in editing the manuscript.
Conflict of interest statement
H. Eltchaninoff and E. Durand declare modest lecture fees from Edwards Lifesciences. M. Abdel-Wahab is a proctor for Boston Scientific. M. Barbanti is a consultant for Edwards Lifesciences and an Advisory Board member for Biotronik and Medtronic.

