
Abstract
Aims: Long-term data on the durability of currently available transcatheter heart valves are limited. We sought to assess four-year clinical and echocardiographic outcomes in patients undergoing transcatheter aortic valve implantation (TAVI) with the CoreValve prosthesis.
Methods and results: Between June 2007 and February 2014, 450 consecutive patients with symptomatic severe aortic stenosis underwent TAVI in our institution. For the purposes of this study, we included only those patients undergoing successful TAVI with the CoreValve prosthesis who had a minimum follow-up of four years (n=125). Survival rates at one, two, three and four years were 83.2, 76.8, 73.6 and 66.3%, respectively. Aortic regurgitation was a common finding after the procedure, especially due to paravalvular regurgitation (PVR), which was observed in the majority of patients (71.5%), mostly mild (52.0%). Progression from mild acute PVR to moderate PVR at four-year follow-up was reported in three patients. No cases of severe PVR were observed. Prosthetic valve failure was reported in four patients (3.2%).
Conclusions: Our study demonstrates that favourable outcomes after successful TAVI are associated with sustained clinical and functional cardiovascular benefits up to four-year follow-up. Signs of moderate prosthetic valve failure are present only in a small percentage of patients.
Abbreviations
AS: aortic stenosis
AVA: aortic valve area
CRS: CoreValve Revalving System
ES: Edwards SAPIEN
MDCT: multi-detector computed tomography
NYHA: New York Heart Association
PVR: paravalvular regurgitation
SAVR: surgical aortic valve replacement
TAVI: transcatheter aortic valve implantation
THV: transcatheter heart valve
VARC: Valve Academic Research Consortium
Introduction
Since the first-in-man transcatheter aortic valve implantation (TAVI) in 2002, more than 100,000 procedures have been performed worldwide, and this intervention has become an accepted and less invasive treatment alternative for high-risk surgical patients. Short- and medium-term outcomes have been encouraging1-5. However, data on long-term clinical outcomes, valve durability, and structural integrity are scarce6-8.
Clinical perspectives on surgically implanted stented bioprostheses in the aortic position have shown ten-year freedom from valvular failure in the range of 60% to 90%9,10. At five years, freedom from structural failure is generally more than 95% and, although early failure requiring reoperation or leading to mortality has been reported, freedom from reoperation at five years is also generally more than 95%11,12. Whether TAVI can lead to similar outcomes is still unknown.
The paucity of evidence supporting long-term durability of currently available transcatheter heart valves is one of the main issues which prevents TAVI being used in younger and lower-risk patients. The aim of this single-centre study was to assess four-year clinical and echocardiographic outcomes in a cohort of patients who underwent TAVI with the CoreValve prosthesis in our institution.
Methods
PATIENT POPULATION
Between June 2007 and February 2014, 450 consecutive patients with severe symptomatic aortic stenosis (AS) underwent TAVI in our institution using the self-expanding CoreValve® (Medtronic, Minneapolis, MN, USA) prosthesis (n=324; 72%), balloon-expandable Edwards SAPIEN (Edwards Lifesciences, Irvine, CA, USA) (n=115; 25.5%) and Lotus™ valve (Boston Scientific, Marlborough, MA, USA) (n=3; 0.66%). For the purpose of the present analysis, only patients undergoing CoreValve implantation from June 2007 to May 2010 and with four-year follow-up available were included (n=125) (Figure 1).
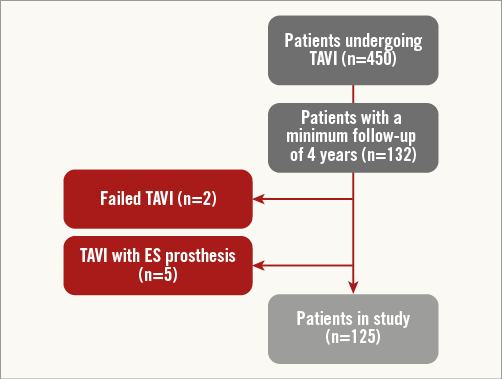
Figure 1. Study flow chart. ES: Edwards SAPIEN; TAVI: transcatheter aortic valve implantation
PATIENT SELECTION
A multidisciplinary team including cardiologists, cardiothoracic surgeons, anaesthesiologists, geriatricians, and interventional cardiologists evaluated all available clinical and imaging data, and a consensus decision was obtained to determine individual eligibility for TAVI. All patients provided written informed consent. The study was approved by an institutional review committee.
Screening studies were performed in all patients before the procedure as previously described13. Sizing of the transcatheter heart valve was carried out by using multi-detector computed tomography (MDCT)14 and a combination of echocardiography (transthoracic and/or transoesophageal), angiography and simultaneous aortography during balloon valvuloplasty13, when MDCT was not available.
DATA COLLECTION AND DEFINITIONS
Patients were enrolled in a prospective database, with clinical evaluations at one month, 12 months and then yearly after TAVI. Transthoracic echocardiography was performed before hospital discharge and then at similar intervals at the implantation centre. For patients located geographically far away from our institution or unable to return to the implantation centre for further studies, follow-up was performed by means of telephone contact. All clinical outcomes were defined according to Valve Academic Research Consortium definitions (VARC-2)15.
ECHOCARDIOGRAPHY
Aortic valve area (AVA) was calculated with the continuity equation (velocity time integral method) from data derived before and after device implantation. Measurement of the left ventricular outflow tract for calculation of AVA was performed with two-dimensional imaging in a zoomed-up parasternal long-axis view.
Prosthetic valve dysfunction was fully evaluated at each follow-up, taking into account prosthetic aortic valve stenosis and paravalvular regurgitation, according to VARC-2 criteria15.
STATISTICAL ANALYSIS
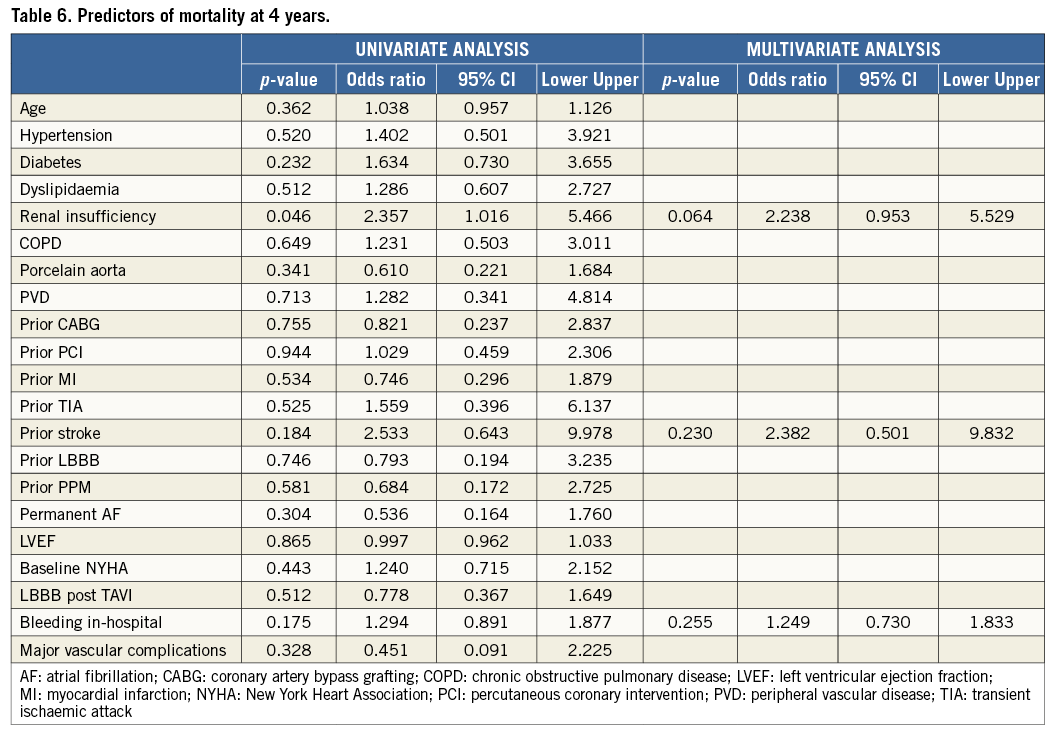
Continuous variables are presented as mean±SD or medians with first (Q1) and third (Q3) quartiles in cases of skewed distributions. Categorical variables are described by frequencies and percentages. Differences between independent groups were tested using the Wilcoxon rank-sum test and Student’s t-test for continuous variables. In cases in which the samples were paired, the Wilcoxon signed rank or paired Student’s t-test was used. Categorical variables were compared with the chi-square test. The cumulative incidences of clinical events at follow-up were assessed with the Kaplan-Meier method and landmark analysis was also carried out for all-cause and cardiovascular death. A Cox multivariate analysis including all variables with probability value <0.20 in each Cox univariate analysis (renal insufficiency, prior stroke and in-hospital bleeding) was used to determine independent predictors of the outcomes. Logistic models were tested in order to detect predictors of mortality at four years. Results were reported in terms of hazard ratio (HR) and 95% confidence interval (CI). All data were processed using the Statistical Package for Social Sciences (SPSS), Version 20 (IBM Corp., Armonk, NY, USA).
Results
POPULATION
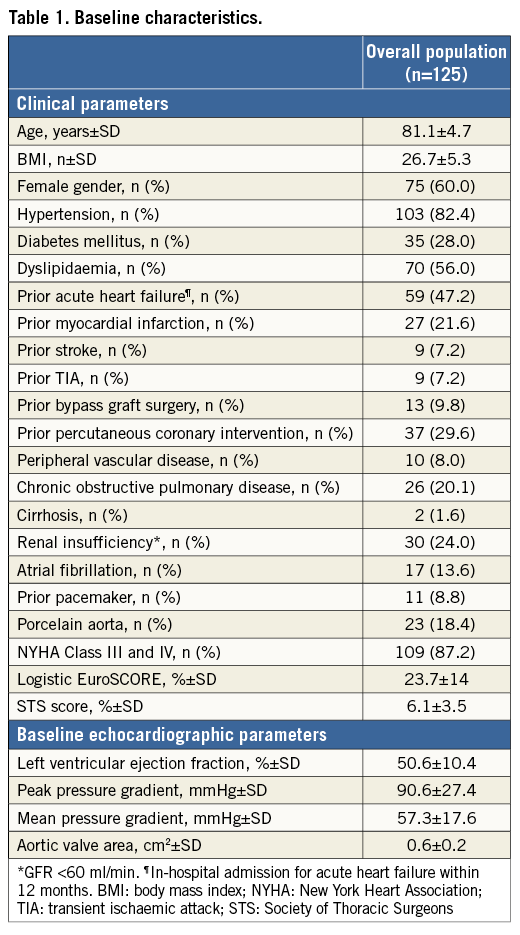
One hundred and twenty-five patients with a mean age of 81.1±4.7 years were analysed. Baseline demographic, clinical and echocardiographic characteristics are summarised in Table 1. All patients had severe symptomatic AS (mean transaortic pressure gradient 57±17.3 mmHg, mean AVA 0.6±0.2 cm2). The predicted 30-day mortality assessed by logistic EuroSCORE and STS mortality score was 23.7±14% and 6.1±3.5%, respectively. The majority of patients (n=109; 87.2%) were in NYHA functional Class I or IV before the procedure.
PROCEDURAL AND IN-HOSPITAL OUTCOMES
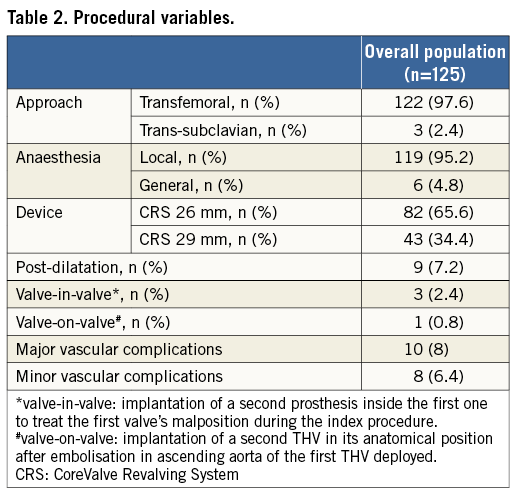
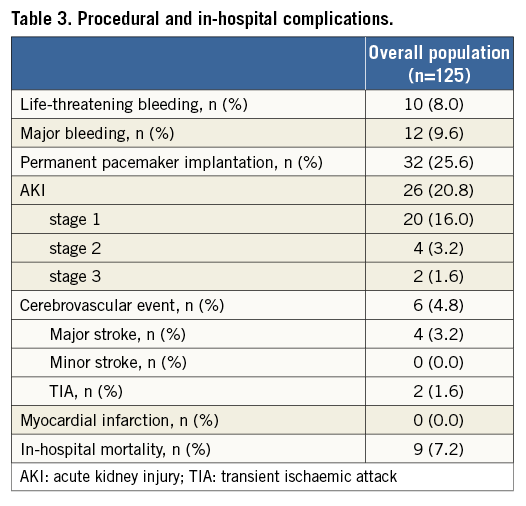
Transfemoral access was used in 122 patients (97.6%); a trans-subclavian access was employed in three patients (2.4%) with an unfeasible transfemoral approach. VARC-defined device success was obtained in 105 patients (84%). Procedural data are shown in Table 2. In-hospital outcomes are listed in Table 3. Overall, in-hospital mortality was 7.2%. A permanent pacemaker was implanted in 25.6% of patients, in most cases due to permanent or intermittent third-degree atrioventricular block. Life-threatening bleeding and cerebrovascular events occurred in six (4.8%) and 10 (8.0%) patients, respectively.
FOUR-YEAR OUTCOMES
Clinical follow-up was available in all patients. A total of 48 (38.4%) patients died during a median follow-up of 52.8 months (IQR 41-62). Survival rates at one, two, three and four years were 83.2, 76.8, 73.6 and 66.3%, respectively (Figure 2A). Survival from cardiovascular mortality rates at one, two, three and four years were 88.0, 84.0, 83.2 and 80.8%, respectively (Figure 2B).
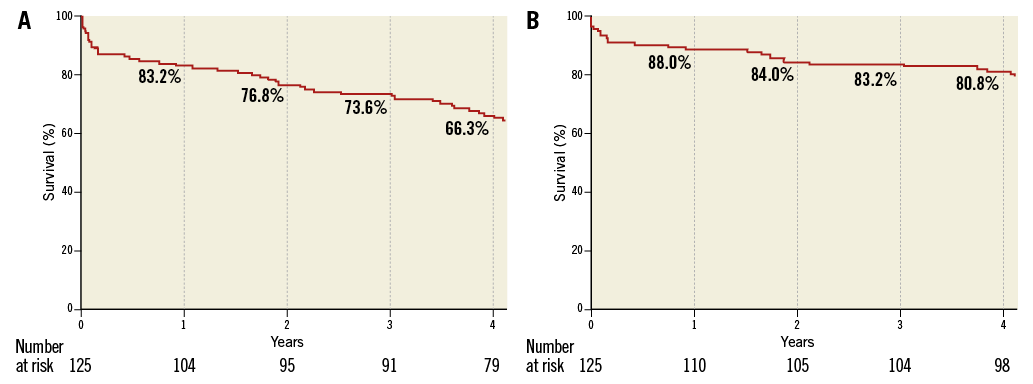
Figure 2. Kaplan-Meier curves of survival from all-cause (A) and cardiovascular death (B) up to 4-year follow-up.
The most common cause of death was due to cardiac reasons (eight deaths, 18.6%), followed by renal failure (five deaths, 11.6%) and bleeding, respiratory and natural causes (four deaths each, 9.3%) (Table 4). Cardiac causes and bleeding were the main causes of death in the early period after TAVI, while the other causes were prevalent at longer follow-up.
When patients who had died at 30 days were excluded, four-year survival from all-cause and cardiovascular death was 72.7% and 85.6%, respectively, as shown by the landmark analysis (Figure 3A, Figure 3B).
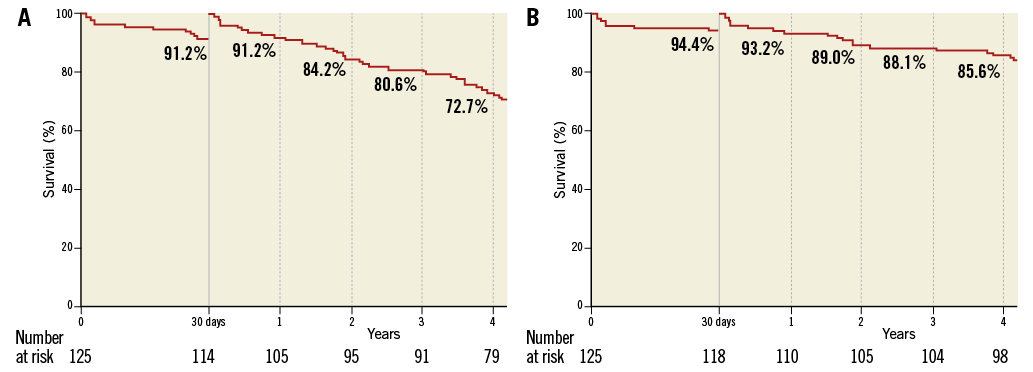
Figure 3. Landmark analysis for survival from all-cause (A) and cardiovascular death (B) up to 4-year follow-up.
The overall neurological event rate at four years was 8.0%, of which more than two thirds occurred early after the procedure. Rates of other VARC-defined complications at follow-up are listed in Table 5. At follow-up, 10 patients (8.0%) required re-hospitalisation due to heart failure. Disabling bleeding and stroke occurred only within 30 days after TAVI. Only one case of myocardial infarction occurred at one-year follow-up.
Overall survival free from reoperation was 98.5%. Only two cases of valve degeneration, both treated by means of implantation of a new transcatheter valve (valve-in-valve), without any subsequent adverse event were reported. The first case was due to a valve endocarditis which occurred one year after the procedure, causing severe aortic regurgitation: it was treated after 92 days from the diagnosis when resolution of the acute phase was confirmed. The second case was a VARC-2 defined moderate/severe restenosis treated after 74 days from the diagnosis.
The NYHA functional Class III/IV at one year was virtually absent in the study population (0.9%). This benefit was sustained at four years (3.3%).
PREDICTORS OF FOUR-YEAR DEATH
At multivariate analysis, renal insufficiency (adjusted HR 2.23, 95% CI: 0.953-5.529; p=0.064) showed a trend towards higher mortality at four years (Table 6).
ECHOCARDIOGRAPHIC FINDINGS
Echocardiographic data were available in 68.3% (56 echo on a total of 82 patients alive at four-year follow-up).
Graphs depicting prosthesis performances at follow-up are shown in Figure 4. Mean pressure gradients decreased from 57±17.3 mmHg (pre-TAVI) to 10.3±4.1 mmHg (in-hospital post-TAVI) (p<0.001). Subsequently, there was a small increase in transaortic gradient at one year (12.5±6.6 mmHg), which remained steady at four years (11.4±6.6 mmHg). The calculated AVA increased acutely from 0.6±0.22 cm2 (pre-TAVI) to 1.5±0.3 cm2 (in-hospital post-TAVI) (p<0.001), and it remained fairly stable over time.
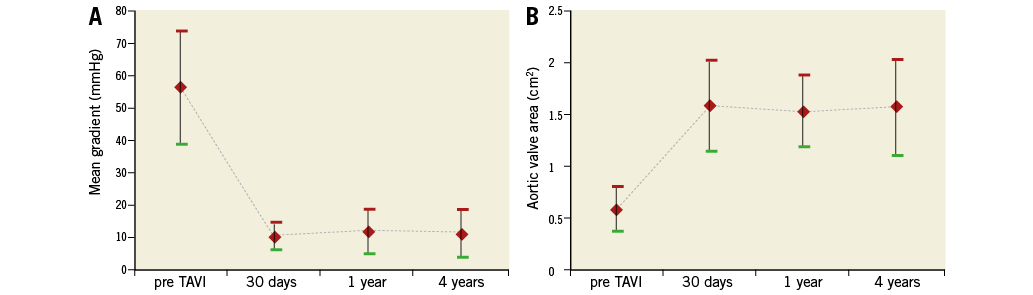
Figure 4. Time trends in transaortic mean gradient (A) and AVA (B).
Aortic regurgitation was a common finding after the procedure, especially due to paravalvular regurgitation (PVR), which was observed in the majority of patients (71.5%), mostly mild (52.0%). At one and four years, moderate PVR was observed in 14 (11.2%) and nine (7.2%) patients, respectively. No cases of severe PVR were noted. Progression from mild acute PVR to moderate PVR at four-year follow-up was reported in three patients.
A total of four patients (3.2%) showed signs of prosthetic valve failure: one patient showed moderate/severe restenosis with a mean gradient of 44 mmHg, another experienced valve endocarditis at two years, causing severe intraprosthetic aortic regurgitation (both cases were effectively treated with redo TAVI as previously described), and two patients had moderate transvalvular regurgitation. These latter patients were asymptomatic and therefore left untreated. Another three patients (2.4%) showed mild stenosis with mean transaortic gradient ranging from 20 to 40 mmHg. No other cases of valvular deterioration were observed. Neither valve thrombosis nor late valve embolisation was reported.
Discussion
TAVI is now considered a valuable alternative to conventional aortic valve replacement in high-risk patients affected by severe AS. While a great number of studies have confirmed encouraging short-term and midterm effectiveness of TAVI1-5, there is still a paucity of data on the benefit of this technique at longer follow-up6-8.
This single-centre analysis adds considerably to the current knowledge of long-term clinical outcomes, valve durability, and structural integrity of percutaneous valves with the following observations: i) successful TAVI showed favourable long-term outcomes with a four-year survival rate of 66.3%; ii) at four-year follow-up, prosthetic valve failure occurred in 3.2% of patients, including one case of valve endocarditis.
CLINICAL OUTCOMES
Contemporary evidence on survival after TAVI at longer follow-up is restricted to a few small studies. In our series of 125 consecutive patients, we found a four-year survival rate of 66.3% with a cardiovascular survival rate of 80.8%. In order to assess the long-term durability of TAVI better, we performed a landmark analysis excluding patients with procedural mortality (death during the first 30 days). Compared with the series published by Toggweiler and colleagues8, we found better survival (72.7% vs. 42%); this discrepancy may be explained by the lower risk profile of patients included in the present analysis.
We observed that cardiac causes and bleeding were the main causes of death in the early period after TAVI as they were more likely procedure-related. However, at longer follow-up, there was an increasing rate of other causes of death, most of them related to patients’ comorbidities and advancing age.
Among major complications, we observed that disabling bleeding and stroke occurred only in the earliest period after TAVI. Finally, hospitalisation due to heart failure during follow-up was required in 8.0% of the entire population.
TRANSCATHETER HEART VALVE PERFORMANCE
As the duration of implanted transcatheter heart valves increases, valve durability and dysfunction become more crucial issues. The durability of transcatheter valves has been a special concern and requires systematic echocardiography follow-up at late time points. Gurvitch et al7 reported on clinical outcomes, valvular structural integrity, and haemodynamic changes in 70 patients evaluated at a median of 3.7 years after TAVI with a balloon-expandable valve, confirming a good medium- to long-term durability and preserved haemodynamic function, with no evidence of structural failure. More recently, Toggweiler and colleagues8 extended the follow-up to five years. In 88 patients (a total of 29 patients alive at five-year follow-up), they demonstrated favourable outcomes after TAVI, with signs of moderate prosthetic valve failure in 3.4% of patients and no cases of severe prosthetic regurgitation or stenosis. Similarly, in the Italian CoreValve registry6, the durability of clinical, haemodynamic and echocardiographic outcomes was tested at three-year follow-up, reporting stable results over the follow-up period. There were no cases of progression of mild PVR to moderate or severe regurgitation.
In the present study, we reported satisfactory long-term valve performance in terms of gradients, AVA and regurgitation. Aortic regurgitation was a common finding after the procedure, especially due to PVR, which was observed in quite a large number of patients (~71%). Consistent with Toggweiler et al, signs of prosthetic valve failure at four years after implantation were observed in 3.2% of population: two patients with new moderate intraprosthetic regurgitation, one case of endocarditis causing severe intraprosthetic aortic regurgitation and one patient with moderate/severe restenosis with a mean gradient of 44 mmHg. The case of severe intraprosthetic aortic regurgitation caused by endocarditis and the restenosis were effectively treated with redo TAVI.
Limitations
The present analysis has two main limitations, the first being the relatively small sample size. However, this represents the largest series of TAVI patients with four-year follow-up published so far. Second, follow-up on echo data was performed in a “survival cohort”, with death possibly exerting a competing risk that may have biased our results. We had 31.7% of surviving patients lost at echo follow-up at four years. Thirdly, we included in the study only patients with a minimum follow-up of four years after TAVI from a population of 450 consecutive patients.
Conclusions
Our study showed that TAVI with the CoreValve prosthesis was associated with sustained clinical outcomes up to four-year follow-up, with a low rate of significant prosthetic valve failure. The procedure appears to be an adequate and lasting resolution to aortic stenosis in selected high-risk patients.
| Impact on daily practice As the duration of implanted transcatheter heart valves increases, valve durability and dysfunction become more crucial issues. The paucity of evidence supporting long-term durability of currently available transcatheter heart valves is one of the main issues which prevents TAVI being used in younger and lower-risk patients. Our study showed that TAVI with the CoreValve prosthesis is associated with sustained good clinical outcomes up to four-year follow-up. Signs of moderate prosthetic valve failure are present only in a small percentage of patients. Neither valve thrombosis nor late valve embolisation was reported. This result confirms the reliability of these prostheses and the good outcomes achieved with TAVI. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

