
Abstract
Aims: The aim of this study was to determine the long-term outcomes of high-risk patients who underwent transcatheter aortic valve implantation (TAVI) with the third-generation CoreValve device, according to the 2017 EAPCI/ESC/EACTS definition of valve durability.
Methods and results: Between 2007 and 2013, 278 consecutive patients were enrolled in our prospective single-centre CoreValve registry (mean age 82±6 years, mean STS score 6.4±5.0%). The median follow-up of survivors was 6.8 years. The Cox proportional hazards model was used to identify independent predictors of HF rehospitalisation and all-cause mortality. Predictors of HF rehospitalisation were LVEF, MR and PVL at the last echocardiographic follow-up. The majority of patients were in NYHA Class I or II and showed mild/trivial paravalvular leak throughout follow-up. Mean pressure gradients remained stable over time. The overall crude cumulative incidences of structural valve deterioration and bioprosthetic valve failure were 3.6% and 2.5%, respectively.
Conclusions: Although overall mortality was high in this elderly patient cohort, the CoreValve bioprosthesis showed good durability at seven-year follow-up.
Abbreviations
AKI: acute kidney injury
BVF: bioprosthetic valve failure
COPD: chronic obstructive pulmonary disease
MPG: mean pressure gradient
PVL: paravalvular leak
STS: Society of Thoracic Surgeons
SVD: structural valve deterioration
TAVI: transcatheter aortic valve implantation
VARC: Valve Academic Research Consortium
Introduction
Transcatheter aortic valve implantation (TAVI) has become the standard of care for high-risk and inoperable surgical patients and a valid alternative in intermediate-risk patients with severe aortic stenosis1-4. The five-year outcomes of the balloon-expandable Edwards SAPIEN bioprosthesis (Edwards Lifesciences, Irvine, CA, USA)5,6, along with the five-year follow-up of the self-expanding CoreValve® bioprosthesis (Medtronic, Minneapolis, MN, USA)7,8, showed good performance with low rates of prosthetic valve degeneration.
The paucity of evidence on the long-term durability of currently available transcatheter heart valves is one of the main concerns that prevents TAVI from being used in younger and lower-risk patients.
Recently, some evidence on longer follow-up of TAVI reporting single-centre experiences has been published9-12.
The purpose of this analysis was to assess the outcomes of TAVI beyond five years, and the durability of the third-generation self-expanding CoreValve prosthesis according to the new European standardised definitions13.
Patients and methods
PATIENT SELECTION
All consecutive patients (n=278) from our centre included in the Clinical Service Project (sponsored by Medtronic Italy; ClinicalTrials.gov NCT01007474) who underwent CoreValve implantation between September 2007 and July 2013 were analysed. Preoperative, intraoperative and postoperative follow-up data were prospectively recorded in a dedicated database.
Decisions on the indication for TAVI and procedural planning were based on the consensus of the Heart Team composed of clinical and interventional cardiologists and cardiac surgeons.
The study complied with the Declaration of Helsinki and was approved by the local ethics committee. All patients provided informed consent.
DATA AND DEFINITIONS
Functional status was graded according to the New York Heart Association (NYHA) classification.
The Society of Thoracic Surgeons predicted risk score (STS score), the logistic European System for Cardiac Operative Risk Evaluation score (logistic EuroSCORE) and the European System for Cardiac Operative Risk Evaluation II (EuroSCORE II) were used to calculate the risk of operative mortality and morbidity. Three classes of operative risk were defined according to the STS score: low (STS <4%), intermediate (STS ≥4% and <8%), and high (≥8%).
Postoperative outcomes were recorded according to criteria of the updated Valve Academic Research Consortium (VARC-2)14.
Structural valve deterioration (SVD) and bioprosthetic valve failure (BVF) were defined according to the EAPCI/ESC/EACTS definitions13.
PROCEDURE AND FOLLOW-UP
The 18 Fr third-generation CoreValve prosthesis, available in the 26, 29 and 31 mm sizes, was implanted using the transfemoral (n=218), trans-subclavian (n=54) or transaortic (n=6) approach. All procedures were performed under fluoroscopic guidance, the majority of them under local anaesthesia (n=225); general anaesthesia and endotracheal intubation were performed in 53 cases. The implantation technique has been described previously15.
Clinical and echocardiographic follow-up data were prospectively collected at discharge, one month, six months and yearly thereafter. Data on survival, NYHA class, adverse events and echocardiographic evaluation were obtained through outpatient visits, telephone interviews, and from collected external medical reports.
In case of suspicion of valve thrombosis based on echocardiography (e.g., leaflet thickening and/or calcification), multi-detector computed tomography was performed.
The date of follow-up census was July 2018.
STATISTICAL ANALYSIS
Categorical variables are presented as frequencies and percentages, and continuous data are expressed as mean±standard deviation (SD).
Survival was assessed using the Kaplan-Meier estimator with curves plotted along with the 95% confidence interval (CI). A log-rank test was used for survival comparisons between groups. The reverse Kaplan-Meier method was used to calculate the median survival time.
Landmark analyses at 30 days and seven years were also performed, and the incidence of the outcomes was assessed using the Kaplan-Meier method at landmark points. The cumulative incidence function adjusted for death-competing risks was used to estimate the incidence of SVD and BVF.
The Cox proportional hazards regression method was used for univariate and multivariate assessment of possible predictors of all-cause mortality and of heart failure (HF) rehospitalisation. Potential covariates in the multivariate analysis were considered because of their statistical significance in the univariate analysis and/or because of their described or supposed clinical relevance.
The hazard ratio is presented with 95% CI. A p-value <0.05 was considered statistically significant.
The SPSS statistical software package, Version 23 (IBM Corp., Armonk, NY, USA), R statistics version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria) and MedCalc version 14.8.1.0. (MedCalc Software, Ostend, Belgium) were used for statistical analysis.
Results
Clinical follow-up was available in all patients (100%). A total of 278 patients (mean age 82.3±5.5 years, mean STS score 6.4±5.0%) were included in the study.
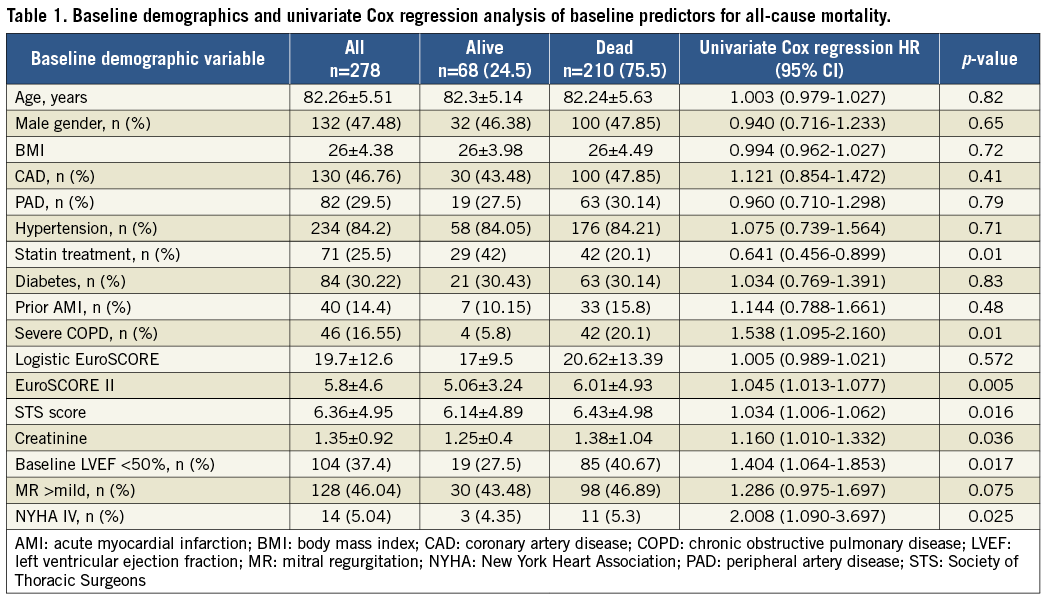
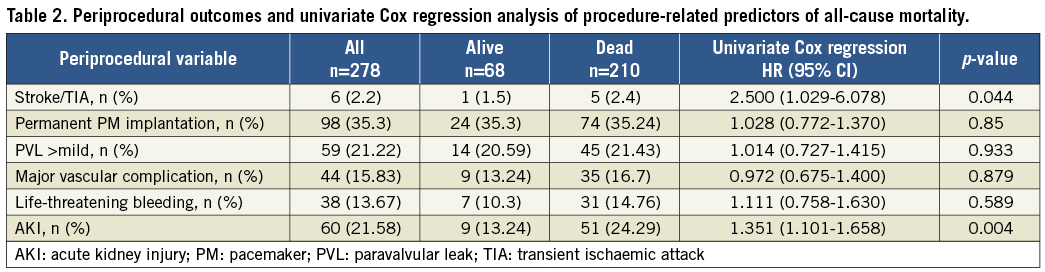
All patients had severe symptomatic aortic stenosis (baseline mean transaortic pressure gradient 51.9±15.5 mmHg). Baseline demographic and periprocedural data are listed in Table 1 and Table 2, respectively.
There were 68 survivors (24.5%) at a median follow-up of 6.83 years, while 210 patients died. The median survival time was 4.7 years (95% CI: 4.3-5.1 years). The longest follow-up was 10.8 years.
The estimated cumulative survival rates at 1, 5, and 8 years were 82%, 45%, and 20%, respectively (Figure 1A). Survival stratified according to STS class of risk showed a stable separation of curves (p=0.02) (Figure 1B). When patients who died within 30 days (7.5%) were excluded, seven-year all-cause mortality was 68.0%, as illustrated by the landmark analyses (Figure 1C, Figure 1D).
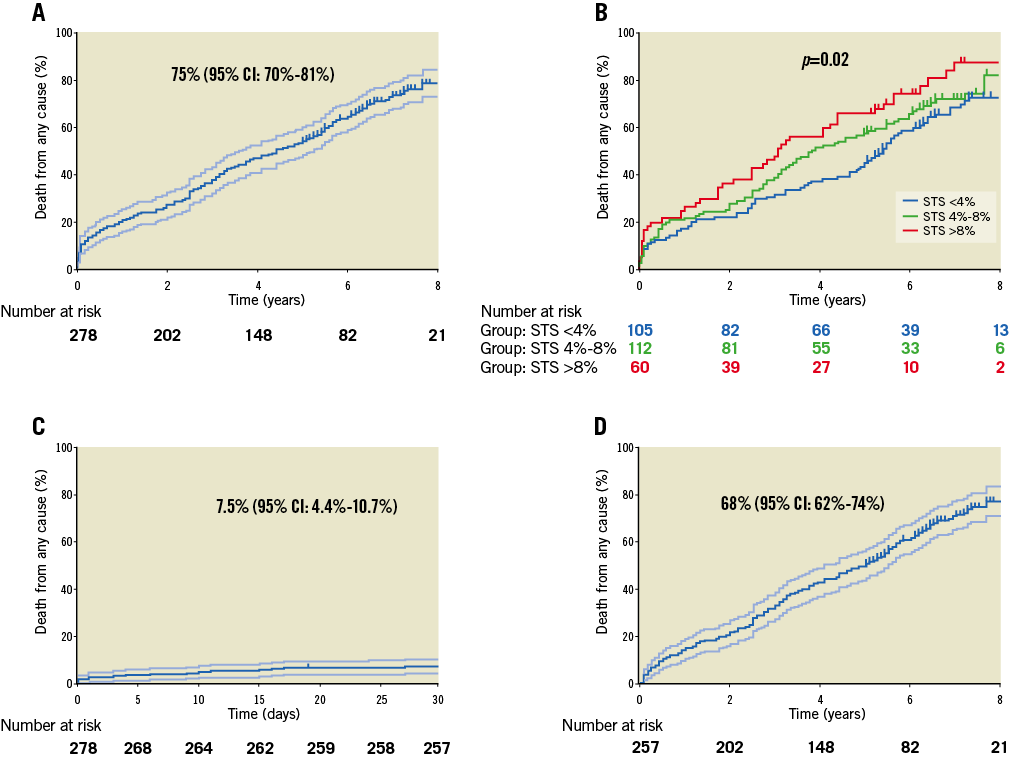
Figure 1. Kaplan-Meier estimates of death. A) Death from any cause plotted with 95% confidence intervals (CI). B) Death from any cause according to STS score classes (blue line: STS <4%; green line: STS ≥4% and <8%; red line: STS ≥8%). Landmark analyses: death from any cause at 30 days (C) and seven years (D) with 95% CI.
Survivors beyond five-year follow-up were 127 (45%), of whom 69 (54%) had an echocardiographic annual assessment (mean echocardiographic follow-up 6.12 years; longest follow-up 9.7 years) (Supplementary Figure 1). This subgroup was composed of patients with a major incidence of prior acute myocardial infarction (AMI), among baseline characteristics, and of cardiovascular rehospitalisations during follow-up. Patients without echo data more frequently had chronic obstructive pulmonary disease (COPD) and left ventricular dysfunction (Supplementary Table 1).
Predictors of all-cause mortality are listed in Table 1, Table 2, and Supplementary Figure 2.
Causes of death in the study population are listed in Supplementary Table 2.
FUNCTIONAL OUTCOME AND PREDICTORS OF HF REHOSPITALISATION
Freedom from cardiovascular rehospitalisation at 1, 5 and 8 years was 80%, 57% and 40%, respectively (Figure 2A). Rehospitalisation due to cardiovascular reasons after discharge occurred in 41% (115) of patients and, among all 157 rehospitalisations, more than half (85) were due to worsening HF (Figure 2B, Supplementary Table 3). Among baseline and periprocedural features, none was significantly associated with increased HF rehospitalisations, both at univariate and at multivariate analysis. Among echocardiographic data available at last follow-up, left ventricular ejection fraction (LVEF) (p=0.04), mitral regurgitation (MR) (p=0.03) and paravalvular leak (PVL) (p=0.04) were independent predictors of HF rehospitalisation at multivariate analysis (Figure 3A, Figure 3B, Supplementary Table 4).
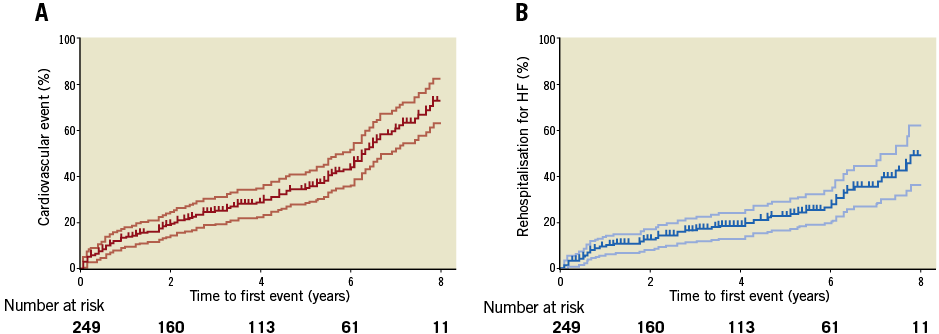
Figure 2. Functional assessment: Kaplan-Meier estimates of rehospitalisation. A) Rehospitalisation for cardiovascular reasons (95% CI). B) Rehospitalisation for heart failure (95% CI) (time to first event).
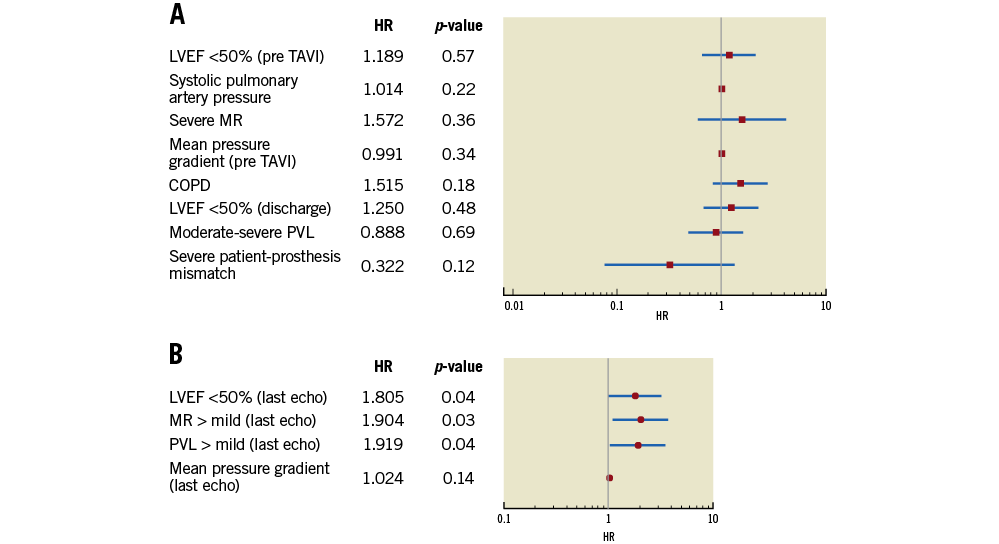
Figure 3. Predictors of HF rehospitalisation. Multivariate Cox regression analysis of (A) baseline and periprocedural and (B) echocardiographic data at last available follow-up.
Significant clinical improvement was demonstrated in the majority of patients who were in NYHA Class I or II early post TAVI and throughout follow-up. At seven-year follow-up, 50% and 42% of surviving patients were in NYHA Class I and II, respectively (Supplementary Figure 3).
HAEMODYNAMIC VALVE PERFORMANCE AND DURABILITY
A complete echocardiographic assessment was available for 23 patients up to seven years of follow-up; fewer patients had longer echocardiographic follow-up available. In the majority of patients, PVL at discharge and throughout follow-up was none/trace or mild. A variable proportion of patients (between 14% and 30%) exhibited moderate PVL throughout seven years of follow-up. Longer follow-up (eight and nine years) showed non-significant PVL (mild or trivial/none), although data were available only for a few patients at these time points. From 0.6% to 1.0% of patients at one-month and two-year follow-up showed severe PVL. The degree of PVL over time is shown in Figure 4A.
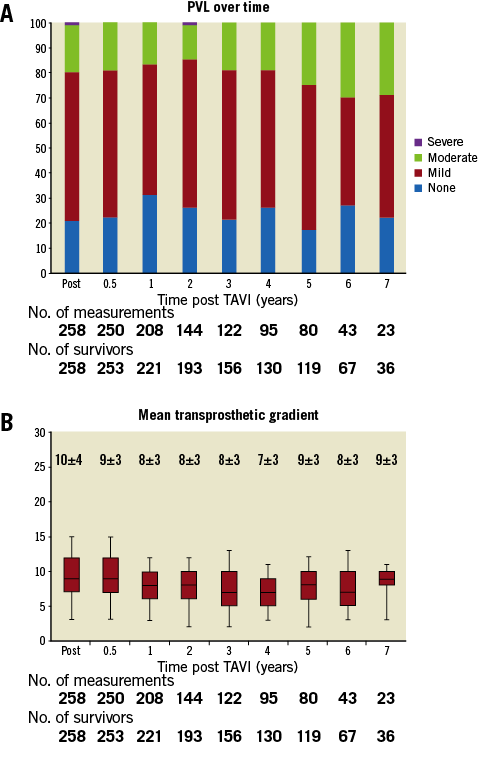
Figure 4. Echocardiographic data. A) PVL over time. B) Mean transprosthetic gradients over time.
Mean pressure gradient (MPG) decreased from 52±16 mmHg (pre TAVI) to 9±5 mmHg (at discharge) (p<0.001). Postoperative MPG remained low and stable throughout follow-up (Figure 4B). At seven and nine years, MPG was 9±3 mmHg and 9±2 mmHg, respectively.
Definite late BVF, defined as severe haemodynamic SVD (>30 days after TAVI procedure) and/or valve dysfunction requiring reintervention, occurred in five patients (1.8%). Of these, three underwent reintervention: one patient underwent successful valve-in-valve implantation for severe stenosis (day 1,693); two patients underwent surgical aortic valve replacement (SAVR), one because of endocarditis (day 858), and one because of severe PVL (day 156). The remaining two patients with prosthesis failure did not undergo further interventions; both patients presented with severe stenosis and regurgitation (day 1,973 and day 2,465, respectively) and died of HF (day 3,569 and day 2,961, respectively).
Finally, two cases of probable BVF (supposed valve-related death) were recorded: one patient with documented moderate stenosis (day 2,193) died because of HF (day 2,288), while another patient with moderate SVD (worsening intraprosthetic regurgitation from mild to moderate at day 555) and HF died at day 1,197.
Three other patients showed moderate SVD, all due to worsening regurgitation from mild to moderate, and died because of non-cardiovascular diseases. Valve thrombosis or late valve embolisation was not observed.
Cases of SVD and BVF are listed in Supplementary Table 5.
At eight years, the overall crude cumulative incidence of SVD and of BVF was 3.6% and 2.5%, respectively. Freedom from BVF at actual analysis (cumulative incidence) was 97.5% and the Kaplan-Meier estimate (actuarial analysis) was 82% at eight years (Figure 5).
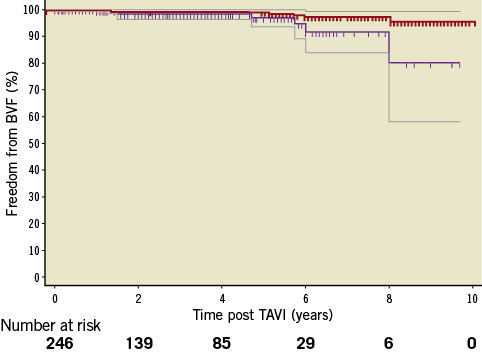
Figure 5. Freedom from BVF. Kaplan-Meier estimate (actuarial analysis) of freedom from BVF with CI (purple lines) and cumulative freedom from BVF adjusted for the competing risk of death (actual analysis, red line).
Discussion
TAVI is recommended in current guidelines as the first choice treatment for patients with aortic stenosis who are at high risk for conventional aortic valve replacement16, based on the results of two randomised clinical trials demonstrating that TAVI is at least non-inferior to surgery at one to three years of follow-up in this patient subset1,2. Although numerous studies have reported favourable short-term and midterm outcomes with TAVI in high-risk patients, few long-term data on durability are available. Moreover, the issue of prosthesis durability is key to extending TAVI to younger patient cohorts at lower surgical risk. Indeed, the 2017 European Guidelines on Valvular Heart Disease propose TAVI as an alternative to surgery in “intermediate-risk” patients, based on recent studies4,16, but caution against the use of TAVI in patients younger than 75 years of age and in low surgical risk patients.
To the best of our knowledge, our study represents one of the largest long-term follow-up studies on the CoreValve prosthesis9,12, with a median follow-up of nearly seven years and a sizeable number of echocardiographic data beyond five years of follow-up.
In our series of 278 consecutive patients, we found a seven-year survival rate of 24.5% which is comparable to that recently reported in a similar population9. The high mortality we observed during follow-up is not surprising, considering the old age and the multiple comorbidities of our population.
We observed favourable long-term clinical outcomes: rehospitalisations due to cardiovascular reasons after discharge occurred in 41% of patients.
The one- and five-year freedom from cardiovascular events was consistent with the rate previously reported7; our study is the first to describe the freedom from cardiovascular events at eight years, which was about 40%. Interestingly, among all studied variables, LVEF, MR and PVL detected at last echocardiographic follow-up were found to be independent predictors of HF rehospitalisation: this result would suggest that, only when these parameters tend to stabilise during follow-up, they may be related with long-term clinical events and gain a prognostic meaning for HF rehospitalisation. It is well known, indeed, that all these factors have shown a variable pattern (stable, improvement or deterioration) in the periprocedural period of TAVI11,15,17.
Moderate-to-severe PVL was associated with an increased five-year cardiovascular mortality, but not with all-cause mortality in the PARTNER 1B trial6. In our study, we confirmed that moderate-to-severe PVL at discharge was not associated with increased overall mortality (Supplementary Figure 2), though significant PVL at follow-up was associated with HF rehospitalisation (Figure 3B).
Finally, concomitant significant MR is usually reported in patients with severe aortic stenosis with discordant results on the clinical effect of baseline MR on outcomes after TAVI17. In our series, the persistence or the occurrence of moderate-to-severe MR during follow-up was associated with a worse long-term clinical outcome (Figure 3B).
VALVE PERFORMANCE
One of the most important findings of this study concerns valve performance that was good at seven years, with SVD observed in only 10 patients.
Notably, only five cases of definite late BVF (1.8%) occurred, in which severe prosthesis dysfunction with clinical correlates was present. Two further cases of probable late BVF were observed.
Toggweiler et al5 reported favourable five-year outcomes after TAVI, with signs of moderate prosthetic valve failure in 3.4% of patients and no cases of severe prosthetic regurgitation or stenosis, according to VARC-1 criteria. Similarly, in the Italian CoreValve registry7, late BVF occurred in five cases (1.4%), as assessed according to VARC-1 criteria.
Gerckens et al8 reported a post hoc analysis on durability in 860 patients after TAVI with a CoreValve bioprosthesis, confirming a good midterm durability according to VARC-2 criteria.
However, those results are not comparable due to the different definitions of SVD and BVF used.
More recently, some authors have reported single-centre experiences beyond five years9-12 defining BVF and SVD according to the new standardised criteria13 and using actual analysis to establish freedom from BVF. Deutsch et al9 reported a cumulative incidence of SVD of 14.9% at seven years: they analysed two different transcatheter heart valves (CoreValve and SAPIEN) with a more favourable outcome for the CoreValve TAVI (11.8% against 22.6%; p=0.01). Although they found a greater incidence of SVD compared to our study, the BVF rate was still low (overall 11 patients [3.6%] and only three cases among CoreValve [1.4%]).
Eltchaninoff et al10 reported a low rate of SVD and of BVF (3.2% and 0.58%, respectively) in a series of 378 patients who underwent balloon-expandable TAVI.
Finally, Holy et al11 reported a cumulative rate of BVF at eight years of 4.5% in a series of 152 patients treated with a CoreValve implantation.
Although the number of patients surviving and with an echocardiographic follow-up beyond five years was relatively small, our study is the largest analysing solely long-term CoreValve behaviour. Furthermore, our study is the first to describe detailed functional outcome at eight years after TAVI, including rehospitalisation for cardiovascular events and for HF and its possible predictors among clinical and echocardiographic variables.
In the present analysis, we report satisfactory long-term valve performance in terms of MPG, which remained steady over time.
Freedom from BVF at actual analysis (cumulative incidence function) was 97.5% and at Kaplan-Meier estimate (actuarial analysis) 82% at eight years.
Several large series have reported the long-term outcomes of surgical bioprostheses, with 10-year freedom from valvular failure in the range of 60% to 95%, depending on prosthesis type and patient characteristics18-20. Importantly, patients undergoing SAVR were on average younger and at lower risk than patients included in TAVI studies, making indirect comparisons of long-term durability inappropriate. Indeed, it is well established that age is inversely related to SVD19. Moreover, some surgical series evaluate durability in terms of survival or freedom from reintervention; others also include worsening of haemodynamic parameters without standardised definitions20,21.
The five-year results of the NOTION trial, comparing TAVR versus SAVR in low-risk patients, presented at the ACC Scientific Session 2018, for the first time showed comparable five-year rates of transcatheter and surgical BVF (7.5% versus 6.7%, p=0.89).
In our study, the occurrence of SVD was not correlated to factors previously reported to be associated with SVD of surgical prostheses, i.e., younger age, higher BMI, patient-prosthesis mismatch, renal failure, dyslipidaemia, smaller annulus and valve size19, because of the small number of SVD observed. Furthermore, there are also fundamental differences between TAVI and SAVR that may impact on the natural history of SVD13. Finally, valve thrombosis and endocarditis have recently been recognised as additional mechanisms leading to transcatheter valve dysfunction22,23.
Although larger studies are needed to determine the rates of SVD and BVF of transcatheter valves at long-term follow-up, our findings demonstrate a favourable durability of self-expanding transcatheter valves at seven-year follow-up.
Limitations
The current study is a non-randomised, observational single-centre study. The results are self-reported with no independent data validation from an external laboratory, which certainly prevents generalisation. The number of patients surviving and with an echocardiographic follow-up of more than seven years was relatively small.
Furthermore, the results may have been influenced by a “learning curve”, considering that our study enrolled the first 278 consecutive patients who underwent CoreValve implantation between 2007 and 2013 in our centre. Greater operator experience, better patient selection and improvements in valve technology in more recent years may favourably affect the long-term outcomes of TAVI. Despite these limitations, the strength of our analysis lies in the clinical and echocardiographic prospective evaluation of outcomes and durability.
Conclusions
TAVI with the third-generation CoreValve device was associated with an overall cumulative incidence of SVD of 3.6% and BVF of 2.5% at seven years. This procedure appears to be a valid and lasting treatment for severe aortic stenosis in high surgical risk patients.
| Impact on daily practice Data on long-term clinical outcomes and durability of TAVI are scarce. Our study shows favourable long-term results with good valve durability at seven years of follow-up. If confirmed in larger studies, these findings may allow the use of this technology in a younger and lower-risk population. |
Conflict of interest statement
A.S. Petronio is a consultant for Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Figure 1. Study flow chart.
Supplementary Figure 2. Multivariate Cox regression analysis (all-cause mortality).
Supplementary Figure 3. NYHA class over time (years).
Supplementary Table 1. Comparison between patients with and without echocardiographic data beyond five-year follow-up.
Supplementary Table 2. Causes of death.
Supplementary Table 3. Causes of cardiovascular rehospitalisation.
Supplementary Table 4. Predictors of HF rehospitalisation: univariate Cox regression analysis of baseline, periprocedural and echocardiographic data at last available follow-up.
Supplementary Table 5. SVD and BVF cases.
To read the full content of this article, please download the PDF.

