
Abstract
Aims: Long-term results of transcatheter aortic valve implantation (TAVI), in particular the incidence of bioprosthetic valve failure (BVF), are uncertain. This study presents data derived from a long-term, structured follow-up programme of the self-expanding CoreValve device utilising standardised definitions and core lab adjudication of valve performance.
Methods and results: The study prospectively included all 152 patients who had undergone TAVI with the self-expanding CoreValve up to December 2011 at the Heart Center, Bad Segeberg, Germany. Late BVF (>30 days) was defined as either: 1) severe structural valve deterioration (transprosthetic mean pressure gradient ≥40 mmHg and/or ≥20 mmHg rise from baseline OR severe intraprosthetic aortic regurgitation), OR 2) bioprosthetic valve dysfunction leading to death or reintervention. Echocardiographic follow-up at 6.3±1.0 years (range: 5.0-8.9 years) was 88% complete (60 out of 68 survivors beyond five years) and all echocardiograms were analysed by an independent core laboratory. The all-cause mortality rate at 1, 2, 5, 6, 7 and 8 years was 14%, 20%, 50%, 60%, 65%, and 73%, respectively. Among survivors beyond five years, effective orifice area was 1.60±0.46 cm2, and transvalvular mean pressure gradient was 6.7±3.1 mmHg; no cases showed evidence of structural valve deterioration. Five patients (3.3%) had undergone redo TAVI (n=4) or surgery (n=1) 0.6 to 5.2 years after the index procedure, all due to paravalvular leakage. The estimated rate of BVF at eight years was 7.9% for the actuarial and 4.5% for the actual analysis.
Conclusions: Long-term follow-up up to 8.9 years after TAVI documents favourable performance of the self-expanding CoreValve with low rates of BVF.
Abbreviations
AR: aortic regurgitation
AS: aortic stenosis
BVF: bioprosthetic valve failure
EOA: effective orifice area
PG: pressure gradient
PVR: prosthetic valve regurgitation
STS: Society of Thoracic Surgeons
TAVI: transcatheter aortic valve implantation
THV: transcatheter heart valve
Introduction
Transcatheter aortic valve implantation (TAVI) is currently the standard of care for patients with severe symptomatic aortic stenosis (AS) and a high operative risk1-3, and is an established alternative to surgery in intermediate-risk patients4. Although the pivotal trials have shown no signals of early bioprosthetic valve failure (BVF) so far, the paucity of data on transcatheter heart valve (THV) long-term durability remains of major concern. Current data on THV performance are limited to five years of follow-up5,6. However, the experience with several surgical bioprostheses has shown that increasing rates of BVF may occur beyond this period7,8. While a durability of at least five years, as suggested by previous studies, is acceptable for an elderly high-risk population, evidence of longer-term durability is mandatory before replacing surgery with TAVI in younger, lower-risk patients. In addition to the relatively short follow-up of current data on THV durability, a number of other shortcomings of current data are worth mentioning: 1) high rates of loss to echocardiographic follow-up among survivors, 2) lack of standardised definition of valve durability criteria, and 3) the application of inappropriate statistical analysis methods, which considers valve dysfunction as a time-dependent variable, overlooking the longitudinal nature of valve degeneration9.
The present study presents data derived from a long-term structured follow-up programme of the self-expanding CoreValve® device (Medtronic, Minneapolis, MN, USA), utilising standardised definitions and core lab adjudication of valve performance9.
Methods
PATIENT POPULATION AND STUDY DESIGN
Since September 2007, all patients undergoing TAVI procedures at the Heart Center, Segeberger Kliniken, Bad Segeberg, Germany, have been included in a prospective registry (NCT03192774) approved by the local ethics committee and conforming to the Declaration of Helsinki.
The current analysis includes 152 consecutive patients who underwent TAVI with a self-expanding transcatheter heart valve system (CoreValve) more than five years before the study (between September 2007 and December 2011). Technical details and implantation techniques have been described previously1. Clinical and echocardiographic follow-up was routinely performed at 30 days, 6 months, and 1, 2 and 5 years. Additionally, for the sake of the present analysis, all patients surviving beyond five years after TAVI were approached and personally interviewed (at the institution or through house visits) for clinical and echocardiographic examinations. Overall, echocardiographic data beyond five years (6.3±1.0 years; range: 5.0-8.9 years) post TAVI were available in 60 out of 68 patients who survived beyond five years post TAVI (88.2%). The reason for incomplete echocardiographic data was death after five years but before a contemporary echocardiography in all eight patients. The study flow chart is displayed in Supplementary Figure 1. Four patients who had undergone a redo valve procedure during the follow-up period were excluded from the echocardiographic analysis of long-term THV performance. The time interval from TAVI to the last echocardiographic follow-up in the remaining 56 patients was as follows: 6th post-TAVI year, n=23; 7th year, n=23; 8th year, n=6; and 9th year, n=4. An independent core laboratory (University Heart Center Zurich, Zurich, Switzerland) blindly analysed all echocardiograms prospectively acquired five years after TAVI. Image interpretation was based on a detailed analysis protocol according to current guidelines and standardised endpoint definitions.
RISK ASSESSMENT AND ENDPOINT DEFINITIONS
Predicted 30-day mortality was estimated according to the logistic EuroSCORE and the Society of Thoracic Surgeons (STS) predicted risk of mortality score. Echocardiographic and clinical endpoints are defined according to the VARC-2 criteria10. The primary endpoints of the study were: a) the rate of late bioprosthetic valve failure (BVF), and b) THV long-term performance (beyond five years). Late BVF was defined according to the recently proposed consensus definition9, including one of the following criteria occurring >30 days after TAVI: 1) severe haemodynamic structural valve deterioration (SVD), evidenced by a transprosthetic mean pressure gradient (PG) ≥40 mmHg and/or ≥20 mmHg rise from baseline OR severe transvalvular aortic regurgitation, OR 2) bioprosthetic valve dysfunction leading to death or reintervention.
STATISTICAL ANALYSIS
Continuous variables are summarised as mean±SD, or as median with interquartile range (IQR), as appropriate. Categorical variables are summarised as frequencies and percentages. Continuous variables were compared using the Student’s t-test or the Mann-Whitney U test. Comparisons between categorical variables were performed using the chi-square or Fisher’s exact test. All tests were two-tailed and a p-value of <0.05 was considered significant. The cumulative incidence of all-cause death and BVF was assessed with the Kaplan-Meier method (actuarial analysis) using GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA). Additionally, for the estimate of freedom from BVF, cumulative incidence (actual analysis) was adjusted for the competing risk of all-cause mortality using the JMP statistics software Version 13.1.0.
Results
STUDY POPULATION, PROCEDURAL DETAILS, AND EARLY OUTCOMES
A total of 152 patients (median age, 81.0 years; STS score, 4.4) were treated with the self-expanding CoreValve THV from September 2007 to November 2011. TAVI was performed predominantly through a transfemoral access (99.3%), and the 26 and 29 mm valve sizes were implanted in 56 (37%) and 96 (63%) patients, respectively. Initial device success was achieved in 107 patients (70.4%) with ≥moderate residual paravalvular AR being the predominant cause of device failure. Baseline and procedural characteristics are summarised in Table 1. The rates of all-cause and cardiovascular mortality and major stroke at 30 days were 6.6%, 4.6%, and 4.5%, respectively. Other 30-day outcomes are detailed in Supplementary Table 1.
LONG-TERM CLINICAL OUTCOMES
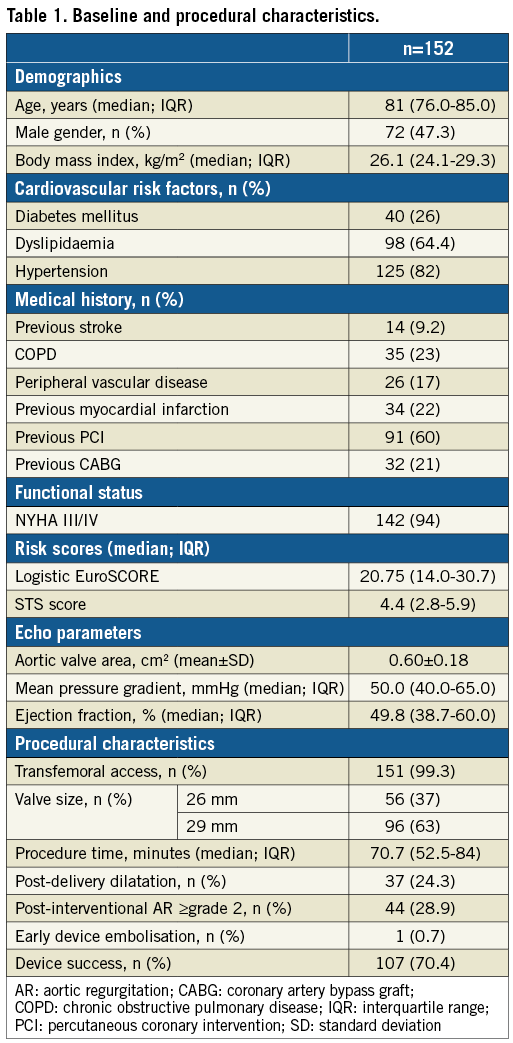
Clinical follow-up was available in 99% of the patients (n=151). A total of 94 (61.8%) patients had died during the period of observation (median time to death or latest follow-up: 5.0 years [range: 0.0-8.9]). Median time to death was 3.4 years (range: 0.0-8.2 years). Rates of freedom from all-cause mortality at 1, 2, 5, 6, 7 and 8 years were 86%, 79%, 50%, 40%, 35%, and 27%, respectively, with a 95% confidence interval for mortality at 7 years of 0.30-0.43 and at 8 years of 0.17-0.39 (Figure 1). Causes of death in the study population are summarised in Supplementary Table 2.
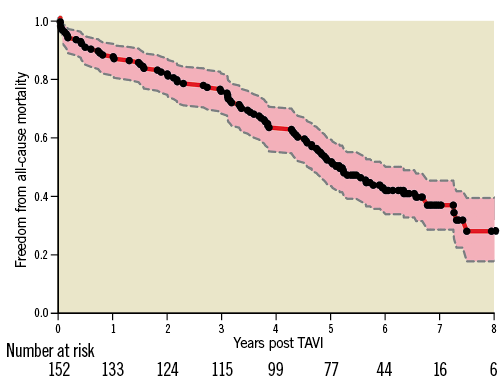
Figure 1. Kaplan-Meier survival curve of the study population. Estimated freedom from all-cause mortality (red continuous line) with the 95% confidence interval (pink area). Black dots represent censored observations.
BIOPROSTHETIC VALVE FAILURE
During the total clinical follow-up period of up to 8.9 years, no severe SVD or death attributable to valve failure was reported. A total of five patients (3.3%) had undergone redo transcatheter (n=4) or surgical (n=1) valve replacement at 0.6 to 5.2 years following the index procedure, all due to moderate-severe paravalvular regurgitation.
Figure 2 displays the estimated freedom from late BVF over a follow-up period of up to 8.9 years according to both actuarial (Kaplan-Meier) and actual analysis (cumulative incidence function). The estimated rate of BVF at both seven and eight years was 7.9% for the actuarial and 4.5% for the actual analysis.
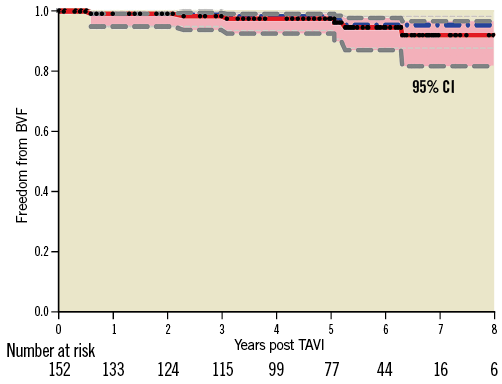
Figure 2. Freedom from BVF after CoreValve implantation. Cumulative freedom from BVF according to the Kaplan-Meier estimate (red line; actuarial analysis, 92.1% at eight years) and adjusted for the competing risk of all-cause mortality (centre dotted blue line; actual analysis, 95.5% at eight years). The pink area demarks the 95% confidence interval. Black dots represent censored observations.
LONG-TERM (≥5 YEARS) PROSTHETIC VALVE PERFORMANCE
In 56 patients with echocardiographic follow-up at 6.3±1.0 years (range: 5.0-8.9 years), effective orifice area (EOA) was evaluable in 50 cases, transvalvular PG in 53, and prosthetic valve regurgitation (PVR) in all cases. EOA averaged 1.60±0.46 cm2 (range, 0.78-3.10 cm2); transvalvular Vmax was 1.80±0.41 m/s (range, 1.10-2.96 m/s); peak PG was 13.7±6.4 mmHg (range, 4.9-35.1 mmHg); and mean PG was 6.7±3.1 mmHg (range, 2.4-18.2 mmHg).
Mean transvalvular PG was 6.3±2.2 mmHg at the 6th year post TAVI (n=22), 6.8±3.5 mmHg at the 7th year post TAVI (n=20), 7.2±4.0 mmHg at the 8th year post TAVI (n=10), and 6.7±3.9 mmHg at ≥9th year post TAVI (n=4). Mean EOA at the 6th, 7th, 8th and 9th year was 1.7 cm2, 1.6 cm2, 1.4 cm2 and 1.8 cm2, respectively.
Vmax was <3 m/s and mean PG was <20 mmHg in all cases and did not increase by ≥10 mmHg from postoperative to latest follow-up echocardiography in any case. EOA was <1.1 cm2 in four patients (range: 0.78-1.06 cm2). These four patients were females with a relatively small body surface area (median, 1.67 m2).
Transvalvular PVR was detected in 6 patients (all mild), paravalvular PVR in 30 (mild in 20, moderate in 9, and severe in 1 patient), and both in 4 patients (mild in 2, moderate in 1, and severe in 1). Overall, total PVR was none-trace in 13 patients (23.2%), mild in 31 (55.4%), moderate in 10 (17.9%), and severe in 2 patients (3.6%).
Moderate-severe PVR at latest follow-up (n=12) was already present at 30-day echocardiography in 2 cases, and progressed from mild in 9 and from none-trace in 1 patient. Compared to 30-day echocardiography, PVR regressed from moderate to ≤mild in 5 patients (Figure 3). Overall, PVR severity remained stable from postoperative to latest follow-up in 27 patients (48%), increased by ≥1 grade in 18 (32%), and improved by ≥1 grade in 11 (20%). Supplementary Table 3 compares echocardiographic findings at 30 days post procedure vs. at late follow-up.
Figure 3. Temporal change in prosthetic valve regurgitation among survivors >5 years post TAVI. Severity of prosthetic valve regurgitation (PVR) in patients with paired transthoracic echocardiographic assessments performed at 30 days and at long-term follow-up (6.4±1.0; range: 5.0-8.9 years) after TAVI.
Discussion
The main findings of the present study of a self-expanding THV are that: 1) the rate of BVF is <8% at long-term follow-up and is mainly due to reintervention for paravalvular regurgitation; 2) there is no signal for early SVD up to eight years post TAVI; and 3) PVR severity tends to change over time in a significant proportion of patients.
The present report is one among few long-term structured and well-documented follow-ups of the performance and durability of the self-expanding CoreValve. Despite the limited number of five-year survivors, the particular strength of this study lies in the high rate of echocardiographic follow-up, the blinded core lab analysis of echo data, and the application of standardised definitions of valve durability and failure.
CLINICAL OUTCOMES
All-cause mortality rates at five years in the current study are comparable to previous studies involving early TAVI patients5,6. Procedure-related complications and stroke were the driving causes of death in the early period up to one year after TAVI, while other conditions related to the patients’ frailty and comorbidities such as congestive heart failure, cancer, infections, falls or unwitnessed events were the predominant causes of death at longer-term follow-up.
THV PERFORMANCE AND BVF
Longitudinal studies with different surgical bioprosthetic valves reported a signal for BVF mostly appearing beyond seven to eight years post implantation7,11. Current evidence on the durability of THV is limited to a few studies reporting short-term and midterm data up to five years only.
A study of a balloon-expandable THV performance (n=70 patients)12 has confirmed a favourable midterm (median, 3.7 years) durability with no evidence of BVF during this relatively limited follow-up period. Another group reported the absence of BVF four years after implantation of a balloon-expandable THV; however, after five years, 9.7% of living patients in that study had moderate prosthetic valve failure, which according to the investigators did not require reintervention5. In the PARTNER I trial, no BVF requiring redo surgical replacement was reported five years after balloon-expandable THV implantation13.
With regard to the self-expanding THV, the Italian CoreValve registry reported stable echocardiographic results over three years, whereas a more recent multicentre analysis described significant prosthesis failure in 1.4% and mild asymptomatic stenosis in 2.8% of the patients over a follow-up period of five years6,14.
The current analysis confirms that transvalvular PG and EOA remain stable in five-year survivors. Mild PVR was common, while ≥moderate PVR was present in 12 patients out of 56 surviving beyond five years and was basically paravalvular. Among the total population (n=151), five patients (3.3%) had a reintervention due to ≥moderate paravalvular PVR. Interestingly, reintervention was in the form of redo TAVI rather than surgery in four out of the five cases. Favourable, yet limited, data are available on the outcomes of redo TAVI, and suggest that TAVI can serve as the default reintervention mode for failing THVs in the future15,16. Similar to our findings, two redo TAVI series identified paravalvular PVR as the most common indication for reintervention after TAVI15,16. Projected rates of BVF at eight years according to the actuarial and actual analysis were as low as 7.9% and 4.5%, respectively.
The rate of ≥moderate paravalvular PVR observed beyond five years after CoreValve implantation is not surprising and in line with earlier, yet shorter, follow-up reports17. The improvement of THV design and the introduction of routine multimodality imaging prior to TAVI has led to a significant reduction in paravalvular PVR rates in contemporary practice. Future longitudinal studies may instead focus on rates of true leaflet deterioration and related clinical events. In the meantime, the present data provide a good reason to believe that SVD of self-expanding valves beyond five years and up to eight years remains rare.
In accordance with some previous reports, we found that PVR tends to change in severity over time in a large proportion of patients18,19. Unlike an earlier report, which suggested that paravalvular PVR after CoreValve implantation tends to regress over time, we did not see a systematic tendency towards improvement19. Rather, we observed a variable pattern (i.e., stable course, improvement, or deterioration). The determinants of such a change should be explored by upcoming studies.
Although no significant rise of transvalvular PG was detected at long term, four patients (8.0%) had a low EOA (≤1.1 cm2). Transvalvular Vmax and peak and mean PG in these patients were median (range): 2.51 (1.8-2.7) m/s, 25.3 (13.0-30.0) mmHg, and 10.6 (5.9-15.8) mmHg, respectively. All cases with reduced EOA at follow-up were de novo (as compared to 30-day data). Whether an isolated reduction of the absolute EOA without a concomitant rise of PG is clinically relevant remains an open question. Notwithstanding, in the single case with markedly reduced EOA (0.78 cm2), left ventricular ejection fraction was markedly reduced (30%). Although this might lead to an underestimation of the EOA, it can also account for the failure of the PG to rise in spite of reduced valvular orifice.
Study limitations
Despite a very close follow-up, the study is derived from a high-risk population with exceedingly high mortality rates and is therefore limited by the relatively low number of patients with a truly long-term follow-up. It is important to underline that assessment of long-term THV performance by echocardiography was only performed through a snapshot examination in a “surviving cohort” and therefore the competing risk of death in this particularly high-risk population may have biased the results. Finally, despite echocardiographic analysis being performed by an independent core laboratory, biases due to measurement variability in this relatively small population cannot be excluded. In line with common clinical sense as well as previous studies, we did not ascribe unwitnessed sudden death to valvular dysfunction, as the latter often manifests as a gradual or a subacute clinical deterioration.
Conclusions
In this long-term clinical and echocardiographic follow-up of patients treated with the self-expanding CoreValve, we found a sustained THV performance with rates of late BVF as low as 4.5% according to currently proposed definitions. The present study cannot conclude – but rather contributes to the growing evidence – upon THV durability.
| Impact on daily practice Long-term results beyond five years after TAVI remain uncertain. The current follow-up (up to 8.9 years after TAVI) documents favourable performance of the self-expanding CoreValve with low rates of BVF. However, further large-scale studies and registries are required to confirm the non-inferiority of THV compared to surgical bioprostheses in terms of long-term durability. |
Conflict of interest statement
M. Abdel-Wahab and G. Richardt report receiving institutional research grants from St. Jude Medical, Biotronik and Medtronic, and lecture fees from Edwards Lifesciences and Boston Scientific. M. Abdel-Wahab is a proctor for Boston Scientific. T. Lüscher has received research and educational grants from Medtronic, St. Jude Medical, Edwards Lifesciences and Biotronik. E. Holy was supported by an EAPCI training grant sponsored by Edwards Lifesciences and by a research grant from the “Walter and Gertrud Siegenthaler” Foundation of the University of Zurich, Zurich, Switzerland. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Figure 1. Study flow chart and patient enrolment.
Supplementary Table 1. 30-day outcomes.
Supplementary Table 2. Causes of mortality.
Supplementary Table 3. Echocardiographic measurements early after TAVI vs. at late follow-up (6.3±1.0 years post TAVI).
To read the full content of this article, please download the PDF.

