
Abstract
Aims: The recently released Medtronic CoreValve Evolut R 34 mm is the largest self-expanding transcatheter heart valve ever developed. Clinical data for this device size are scarce. We therefore aimed to evaluate the clinical performance and safety of the new device.
Methods and results: We report on the first 101 consecutive patients treated with transfemoral transcatheter aortic valve implantation (TAVI) using the 34 mm Evolut R device in a multicentre registry. Clinical parameters were determined before the procedure and echocardiography was performed at baseline and discharge. VARC-2 criteria were assessed at 30 days. Mean age was 80.7 years; mean logistic EuroSCORE was 19.8%. Procedural duration was 71.6 min. Echocardiography at discharge revealed a mean AVA of 2.0 cm2, moderate aortic regurgitation (AR) in 4.0% and severe AR in 1.0%. VARC-2 device success was achieved in 92.1%, while the early safety endpoint occurred in 11 patients (10.9%). New permanent pacemakers were implanted in 17 patients (18.7%). Thirty-day mortality was 2.0%, a stroke occurred in 3.0%, with a disabling stroke in one patient. The incidence of major vascular complications and bleeding was 1.0% and 5.0%, respectively.
Conclusions: Initial experience with the new self-expanding CoreValve Evolut R 34 mm valve is characterised by high procedural success, good haemodynamic performance and a low early complication rate.
Abbreviations
AR: aortic regurgitation
AS: aortic stenosis
AVA: aortic valve area
MPG: mean pressure gradient
MSCT: multislice computed tomography
PPI: permanent pacemaker implantation
PVL: paravalvular leak
TAVI: transcatheter aortic valve implantation
VARC-2 Valve Academic Research Consortium-2
Introduction
Current guidelines recommend TAVI in inoperable and high-risk patients with severe symptomatic aortic stenosis (AS) as well as in intermediate-risk AS patients, particularly when transfemoral access is feasible1. TAVI valve design has undergone rapid evolution with first-generation devices hallmarked by several limitations such as lack of true repositionability, the occurrence of significant paravalvular leaks (PVL), conduction defects and/or major access-site complications2.
The Medtronic CoreValve® Evolut™ R family of valves belongs to the second generation of self-expanding transcatheter heart valves (THV), characterised by several changes in valve design such as optimisation of annular sealing and the option to reposition the valve before full release3,4.
The recently released CoreValve Evolut R 34 mm (Medtronic, Minneapolis, MN, USA), CE marked in January 2017, currently represents the largest available THV, covering annulus perimeters up to 94.2 mm, which relates to an annulus diameter of 30 mm compared to an annulus diameter (area-derived) of 29.5 mm in its direct competitor, the 29 mm Edwards SAPIEN 3 (Edwards Lifesciences, Irvine, CA, USA). In contrast to its smaller relatives (14 Fr), the 34 mm EnVeo™ R delivery system utilises a 16 Fr-equivalent InLine™ sheath (both Medtronic). Moreover, compared to its predecessor, the CoreValve 31 mm, the Evolut R 34 mm features a wider inflow to cover larger annuli, a shorter length and outflow width as well as a more gradual inflow angle to maintain target oversizing of deeper implants.
Here we report on the first real-life experience with this device in 101 consecutive patients treated in five centres in Germany.
Methods
PATIENT POPULATION
The objective of this registry was the evaluation of clinical performance and safety of the new device. The trial was approved by the ethics committee at Kiel University, Germany. The sole inclusion criterion besides the use of the CoreValve Evolut R 34 mm valve was the decision for TAVI made by the local Heart Team. Multislice computed tomography (MSCT) scan analyses were used as the basis for implantation planning. The choice of prosthesis was made by the Heart Team of each of the five participating centres individually. Factors such as relevant annulus eccentricity and LVOT calcifications favoured the decision to choose a self-expanding valve prosthesis. MSCT data were analysed using the 3mensio (Pie Medical Imaging, Maastricht, the Netherlands) or syngo.CT Cardiac Function - Valve Pilot (Siemens Healthcare, Erlangen, Germany) software.
DATA COLLECTION AND STUDY ENDPOINTS
Patient baseline data were collected at admission, and echocardiography was performed before TAVI and at discharge. PVL was assessed during the implantation procedure by angiography by operator eyeballing. Before discharge the PVL was documented by echocardiography at the individual centres. Echocardiographic and angiographic data were analysed without using a core lab. Procedural characteristics, complications and 30-day efficacy and safety outcomes were recorded according to VARC-2 criteria5.
TAVI PROCEDURES
The implantation procedure was carried out as described before3. All valves were implanted using a fast pacing regimen (100-140/min). Predilation and/or post-dilation and choice of general or local anaesthesia were left to the implanting team’s discretion. Arterial closure at the access site was typically achieved by utilising Perclose ProGlide® devices (Abbott Vascular, Santa Clara, CA, USA).
STATISTICAL ANALYSIS
Statistical analyses were performed using the SigmaStat® 3.5 software (Systat Software, Inc., San Jose, CA, USA). Continuous variables were described as mean ±SD. Categorical variables were expressed using percentages.
Results
PATIENT CHARACTERISTICS
A total of 101 patients were treated with TAVI using the CoreValve Evolut R 34 mm system at five centres. The majority of the patients were male (92.1%). The mean Society of Thoracic Surgeons (STS) score was 4.9%. The logistic EuroSCORE and the EuroSCORE II averaged 19.8% and 5.4%, respectively. Before treatment, 83.2% of the patients suffered from dyspnoea NYHA Class III or IV (Table 1).
The mean pressure gradient (MPG) averaged 41.7 mmHg. The cohort included four patients with severe aortic regurgitation (AR). Mean MSCT-derived annulus perimeter was 87.5±5.2 mm (Supplementary Table 1).
PROCEDURAL OUTCOMES
The mean procedural duration was 72.1 min, ranging from 31 min to 355 min. In the longest procedure, TAVI was followed by a conventional valve replacement, the only case with bail-out surgery. Balloon valvuloplasty was carried out in 55.4% before TAVI. After initial valve deployment, moderate or severe AR was evident in 35.6% of the cases (36 patients) and post-TAVI balloon valvuloplasty was performed in 47 patients (46.5%); 5.0% of the patients had moderate (four patients) or severe (one patient) AR as a final result. Four patients received a second valve as a bail-out TAVI procedure within 30 days (Supplementary Table 2).
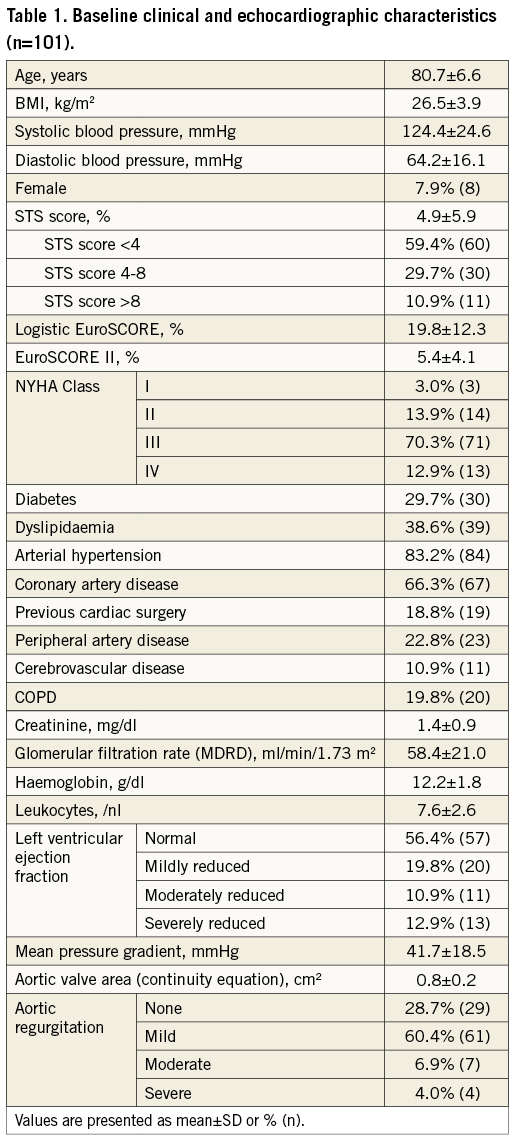
Echocardiography before discharge revealed moderate or severe AR in 5.0%. None or mild AR was evident in 93% (Table 2). Mean aortic valve area improved from 0.8 cm2 to 2.0 cm2, whereas MPG was reduced from 41.7 mmHg to 7.6 mmHg (Figure 1).
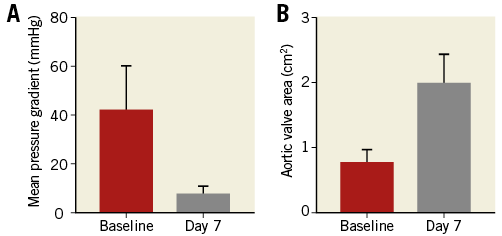
Figure 1. Echocardiographic results. A) Mean pressure gradient (MPG) before procedure and at discharge (seven days post procedure). B) Calculated aortic valve area before procedure and at discharge (seven days post procedure). Data are presented as mean±standard deviation.
At discharge all patients were alive.
CLINICAL OUTCOMES AT 30 DAYS
Clinical outcomes were assessed according to the Valve Academic Research Consortium-2 (VARC-2) criteria at 30 days. Two patients died during this period, one after ischaemic stroke following TAVI and bail-out conventional surgical replacement, the other from pneumonia. Overall, ischaemic stroke occurred in 3.0% of the patients, one of which was a disabling stroke. Major bleeding was detected in 6.9% of the cases. Major vascular access-site complications occurred in one patient, while minor vascular access-site complications were evident in 5.0% of the cohort. Conduction disturbances and arrhythmias occurred in 25.7% of the patients, resulting in permanent pacemaker implantation in 18.7% (excluding patients with pre-existing devices) (Table 2).
According to VARC-2, device success was achieved in 92.1% of the cohort, while early safety endpoints were detected in 10.9% of the study population.
Discussion
Here we report the largest series to date of patients receiving a TAVI procedure with the new Medtronic CoreValve Evolut R 34 mm bioprosthesis.
The study population represents a heterogeneous TAVI patient cohort with a risk profile bearing a mean STS score of 4.9%, 59.4% being low-risk patients, and a mean age of 80.7 years.
Overall, this clinical registry demonstrates favourable early outcome with a 30-day mortality rate of 2% and a disabling stroke rate of 1%. These results compare well to those of the FORWARD registry which analysed the results from 1,038 patients treated with CoreValve Evolut R valves (23/26/29 mm)6. This registry reported a 30-day mortality rate of 1.9% and a major stroke rate of 1.8% in a cohort with comparable risk profile (mean STS score 5.5%). We achieved low transvalvular gradients (MPG 7.6 mmHg relating to a calculated effective orifice area of 2.0 cm2), in line with measurements obtained in SURTAVI (mean MPG at discharge of 8.9 mmHg)7, FORWARD (MPG 8.5 mmHg)6 and the Evolut R CE mark trial (MPG 9.2 mmHg)3.
Five percent (5.0%) of our patients showed moderate (four patients) or severe PVL (one patient), similar to the 5.3% of moderate or severe PVL seen in the Evolut R U.S. study8. However, in the FORWARD registry, a rate of moderate or severe PVL of only 1.9% was observed at discharge6.
Compared to other registries evaluating the CoreValve Evolut R in smaller sizes, we here report higher numbers of second valves used (4%). Bail-out procedures were necessary because of moderate/severe AR due to PVL caused by heavy leaflet or LVOT calcifications and/or leaflet destruction by balloon valvuloplasty. One surgical bail-out procedure was necessary due to dislocation of the prosthesis into the LVOT resulting in severe AR.
Murray and co-authors described a 35% rate of permanent pacemaker implantation (PPI) using the CoreValve 31 mm compared to 18.7% observed in our Evolut R registry9. While the CHOICE trial found a significant difference in PPI comparing two first-generation devices, the CoreValve (self-expanding, PPI 37.6%) and the SAPIEN XT (Edwards Lifesciences) (balloon-expandable, PPI 17.6%)10, the second-generation valve types appear to show a lesser difference in PPI. Husser and colleagues recently described a very low PPI rate of 9.9% in another self-expanding valve (ACURATE neo™; Symetis/Boston Scientific, Marlborough, MA, USA) and 15.5% for the balloon-expandable Edwards SAPIEN 311. Our results for PPI thus confirm the positive trend for the CoreValve Evolut R family with an 11.7% PPI rate in the 26/29 CE mark trial3.
Despite the rather complex “sheathless” implant technique involving several sheath exchanges3, we found a very low rate of access-site complications12. The high number of male patients (92.1%) with presumably large access vessels might have contributed to the low rate of major and even minor bleeding complications.
When comparing the combined VARC-2 endpoints from our registry with the results obtained in the CoreValve Evolut R CE mark trial3, the device success rate with the 34 mm system (92.1% vs. 78.6%) is higher, while VARC-2 early safety endpoints (10.9% vs. 13.3%) are similar between valve sizes.
Limitations
The retrospective character of the study, the sample size of 101 patients and the lack of a core lab represent potentially relevant limitations. A significant strength of this study is the inclusion of the first consecutive patients at five large TAVI centres under rigorous use of the VARC-2 criteria.
Conclusions
This consecutive registry of the first 101 patients treated at five high-volume centres with the new Medtronic CoreValve Evolut R 34 mm TAVI device confirms the encouraging efficacy and safety results of the established smaller CoreValve Evolut R valves.
| Impact on daily practice The use of the new Medtronic CoreValve Evolut R 34 mm TAVI device is feasible, safe and effective in a broad cohort of patients. |
Conflict of interest statement
C. Kuhn received speaker’s honoraria from Medtronic. C. Frerker works as a proctor for Medtronic. U. Schäfer works as a proctor for Medtronic, and has received travel support as well as speaker’s honoraria from Medtronic. M. Abdel-Wahab reports receiving institutional research grants from St. Jude Medical, Biotronik, and Medtronic, and works as a proctor for Boston Scientific. D. Frank works as a proctor for and has received speaker’s honoraria as well as travel support from Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Table 1. Multislice computed tomography data (n=87).
Supplementary Table 2. Procedural outcomes (n=101).
To read the full content of this article, please download the PDF.

