Abstract
Aims: The CoreValve Revalving System (CRS) (Medtronic Inc., Minneapolis, MN, USA) is currently available in four sizes: 23 mm, 26 mm, 29 mm and 31 mm. The aim of our study was to assess the acute clinical results after implantation of a 31 mm CRS.
Methods and results: We assessed device safety, procedural success and 30-day outcomes of 76 consecutive patients who underwent transcatheter aortic valve implantation (TAVI) from January 2012 to September 2013, for severe aortic valve disease. The device sizes used were 26 mm in 21 (28%), 29 mm in 20 (26%) and 31 mm in 35 (46%) patients. Patients who received the 31 mm CRS were significantly younger, predominantly male, with higher STS scores and lower left ventricular ejection fraction. Device success was obtained in 70 cases (92%) in the overall population, without significant differences among groups. Procedural, fluoroscopy and revalving time and procedural outcomes did not differ. A significant reduction in transaortic gradient was obtained in all groups. We observed a low incidence of paraprosthetic leak in the 31 mm group. Permanent pacemaker implantation, bleeding, vascular and renal complications were similar among groups. Time of hospitalisation in CRS 31 mm patients did not differ from the other groups and 30-day outcomes were comparable.
Conclusions: The 31 mm CRS can be safely implanted in patients with complex aortic valve disease, large annuli and dilated left ventricles.
Introduction
Transcatheter aortic valve implantation (TAVI) is a validated treatment modality for severe aortic stenosis (AS) in patients who are inoperable or at high surgical risk1.
The CoreValve Revalving System (CRS) (Medtronic Inc., Minneapolis, MN, USA) has been in clinical use since 2007. Initially it was available in two sizes (26 mm and 29 mm) based on the diameter of the inflow portion of the stent. This was followed by the introduction of 31 mm and 23 mm devices2.
The 31 mm device has been developed for larger annuli (Figure 1). The availability of an additional larger device has permitted the use of TAVI in challenging aortic root anatomy, dilated left ventricle or severe native aortic regurgitation (AR). There are no significant data on the usage pattern of the CRS prosthesis after the introduction of the 31 mm device size.
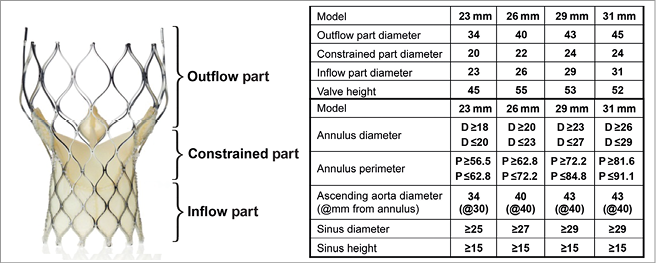
Figure 1. Characteristics of the CoreValve Revalving System prosthesis (Medtronic).
We report on the immediate and 30-day outcomes of TAVI performed in our institution after the introduction of the 31 mm CRS prosthesis.
Methods
STUDY DESIGN
We analysed data from a dedicated database of TAVI procedures performed consecutively at our institution in patients with aortic valve disease from January 2012 to September 2013 with emphasis on aortic root measurements, anatomic criteria for device size selection, technical issues encountered during implantation, device safety, procedural success and early follow-up at 30 days.
DEVICE DESCRIPTION
The prosthesis frame comprises three distinct regions that interact with the aortic root at different levels (Figure 1). The lowermost inflow portion has high radial strength to facilitate device anchoring. The constrained middle portion incorporates the valve leaflets and has the smallest diameter to avoid coronary ostial jailing. The outflow portion serves to orient the frame to the direction of blood flow in the ascending aorta.
PATIENT SELECTION
All patients underwent a multidisciplinary clinical evaluation with transthoracic and transoesophageal echocardiography (TTE, TEE) and multislice computed tomography (MSCT) with multiplanar reconstructions of the left ventricular outflow tract (LVOT), aortic valve, and aortic root, as previously described3,4. Aorto-iliac and femoral arteries were assessed with CT scan and angiography. All patients had severe symptomatic aortic valve disease (stenosis and/or regurgitation) and were at high risk for surgical aortic valve replacement. The assessment of surgical risk was based on a consensus by a multidisciplinary Heart Team meeting, using the logistic EuroSCORE, STS score and relevant comorbidities5. In patients where the femoral approach was not suitable, distal left transaxillary artery access with surgical exposure was used for vascular access. Informed consent was obtained from all patients.
DEFINITIONS AND CLINICAL OUTCOMES
Device success, clinical efficacy and 30-day safety were assessed in the overall population using the VARC-2 criteria5.
STATISTICAL ANALYSIS
Continuous variables following a normal distribution are presented as means with standard deviations and were compared using Student’s t-tests. Categorical variables are presented as counts and percentages. A two-sided p-value ≤0.05 was considered of statistical significance. All data were processed using SPSS 18 (SPSS Inc., Chicago, IL, USA).
Procedure
DEVICE SELECTION
The choice of device size was based on measurements obtained with echocardiography and MSCT: annular diameters and perimeter just below the nadir of the insertion of aortic cusps after reviewing the table supplied by the manufacturer (Figure 1). The annulus perimeter was mainly considered, but aortography performed during balloon aortic valvuloplasty (BAV) was used as an additional tool to confirm device size selection.
In cases where the measured annular diameters were in the borderline zone for 26 or 29 mm valves, the sinus of Valsalva (SoV) diameter (width) was considered, to account for compression of native aortic valve leaflets from the inflow portion of the device. In cases of SoV ≤28 mm, the 26 mm device was used. In patients with dilated left ventricle, when AR was the only or predominant valve malfunction, or in case of aortic root diameters in the borderline zone, we opted for the larger prosthesis. For valve-in-valve procedures on a degenerated surgical aortic bioprosthesis, the device size was based on the inner diameter of the aortic prosthesis6. When a suboptimal position with low implant and significant paraprosthetic regurgitation occurred, a second valve of the same size was implanted (TAV-in-TAV).
VASCULAR ACCESS PREPARATION
All TAVI procedures were performed under local anaesthesia and analgesia with conscious sedation. The percutaneous transfemoral approach for TAVI was performed in a standard fashion, as previously described in detail7. In patients where femoral access with the 18 Fr introducer was contraindicated, the left distal axillary artery with surgical exposure was used as an alternative route8.
IMPLANTATION TECHNIQUE
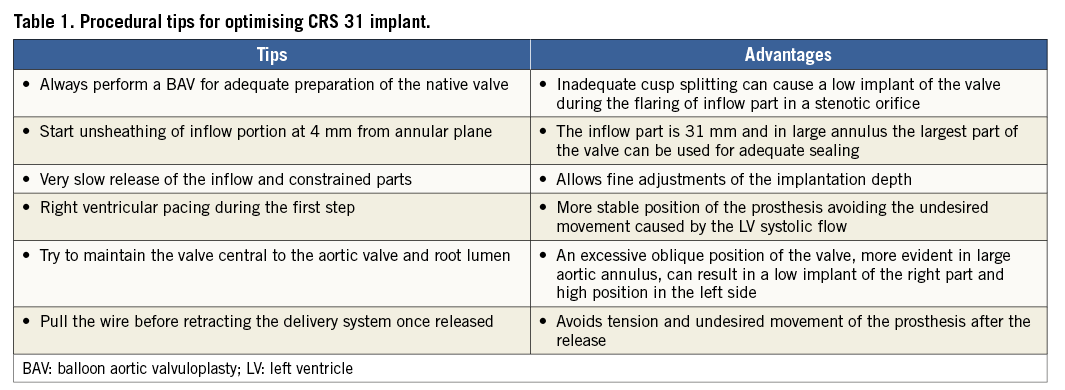
After crossing the native aortic valve with a standard diagnostic Amplatz Left (AL-1) catheter and straight tip wire, an SSA-1 wire with a hand-shaped pigtail loop at the end was placed in the left ventricular apex in a stable position using the right anterior oblique projection. A pre-implantation BAV was routinely performed under rapid right ventricular pacing with an undersized balloon (1-2 mm smaller than the measured aortic annulus diameter) for preparing the native annulus in all cases except for pure AR or degenerated bioprosthesis. The CRS implantation was performed using the standard three-step release technique. A pigtail catheter was positioned in the non-coronary cusp as a marker for the annular plane and for contrast injections during the valve release. The image intensifier was positioned at the implant angle defined as the optimal left anterior oblique (LAO) projection for aligning the nadir of all three coronary cusps in a straight line. The delivery catheter system (DCS) was positioned such that the horizontal markers of the device were positioned below the level of the pigtail catheter. The DCS was maintained as perpendicular to the annular plane as possible, and the release was initiated under fluoroscopic and angiographic guidance with repeated small contrast injections (10 cc/sec at 900 psi) through the pigtail catheter. The salient technical expedients and their underlying rationale are listed in Table 1. Briefly, 1) the DCS frame is positioned 4 mm below the annular plane before starting to release the valve frame; 2) the release of the inflow portion was performed very slowly making small adjustments to compensate for undesired movement; 3) the DCS was maintained as centrally as possible in the aortic root during the deployment, avoiding its preferential alignment along the outer curvature of the aortic root; 4) in all cases of 31 mm CRS implant the first two steps were performed under rapid ventricular pacing (150-200 bpm) which was sufficient to reduce the systolic blood pressure below 60 mmHg (Moving image 1). Pacing was started just at the beginning of device flaring and continued till the entire inflow portion was flared. This manoeuvre helped to maintain the centrality of the DCS in the aortic root and control the depth of implantation in the LVOT during landing of the inflow portion under the left aortic cusp. During the final phase of deployment, the stiff wire was pulled backed a few millimetres to release the tension in the system while the DCS was pushed forward to avoid undesired device displacement towards the aorta (Moving image 2). After the prosthesis was released completely, the release hooks of the device were carefully observed to ensure their detachment from the DCS in LAO and right anterior oblique (RAO) projections. Post deployment, haemodynamic measurements and aortic angiography in two orthogonal projections were performed to assess for the presence and severity of paraprosthetic regurgitation (Moving image 3).
Results
PATIENT DEMOGRAPHICS
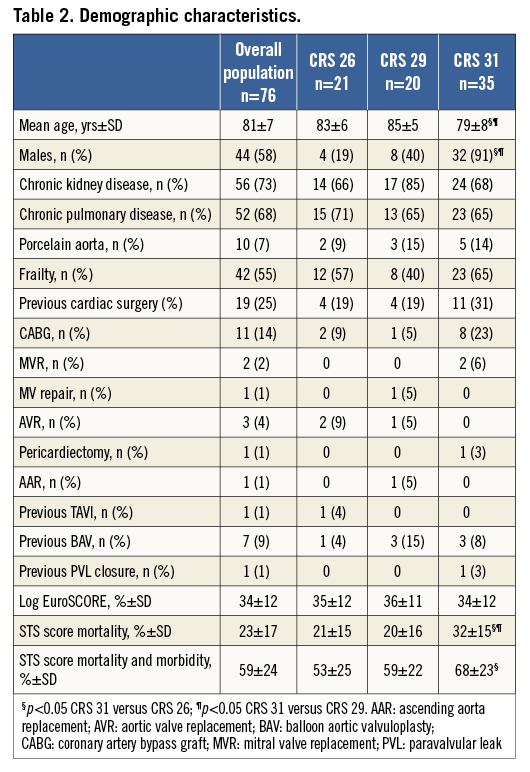
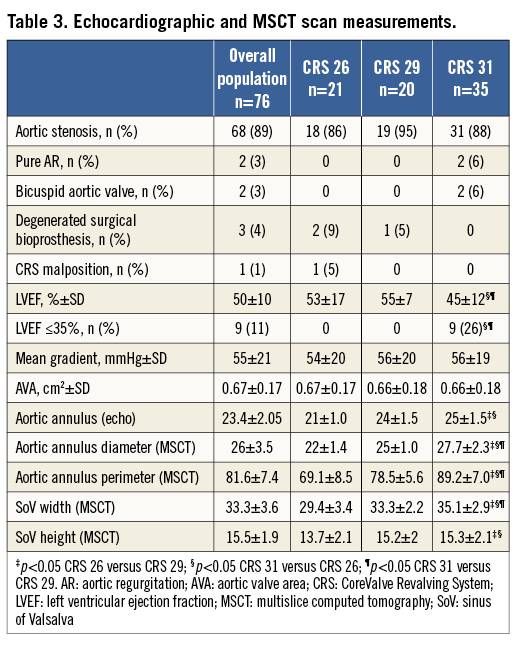
From January 2012 to September 2013, 76 consecutive patients (58% male; mean age 81±7 years) underwent TAVI in our institution. All patients had symptomatic severe aortic stenosis (aortic valve area 0.67±0.17 cm2; mean gradient 55±21 mmHg), except for two patients with pure severe AR, three with degenerated aortic surgical bioprostheses and one patient who underwent a repeat TAVI after a prior CRS implantation performed in another centre and complicated by low malposition. Clinical data are summarised in Table 2; echocardiographic and MSCT measurements are reported in Table 3.
PROCEDURAL OUTCOMES
The procedural outcomes are reported in Table 4. All interventions were completed without general anaesthesia except for one patient who required endotracheal intubation for intraprocedural systemic desaturation. All patients were awakened in the catheterisation laboratory at the end of the procedure.
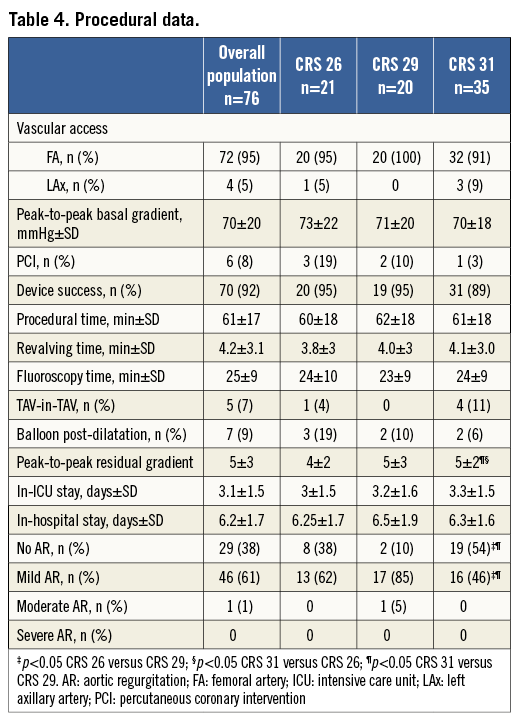
The device sizes used were 26 mm CRS in 21 (28%), 29 mm in 20 (26%) and 31 mm in 35 (46%) patients. Device success was obtained in 70 cases (92%). One case of moderate AR after CRS 29 mm implantation was observed; in five patients a second CRS was implanted, in the same procedure, using the TAV-in-TAV technique, because of haemodynamically significant paraprosthetic regurgitation due to low implantation of the first device, with immediate resolution of the insufficiency and haemodynamics. In seven patients a balloon post-dilatation was needed after CRS implantation to treat underexpansion of the device secondary to annular calcium. Six patients (8%) underwent planned intraprocedural percutaneous coronary intervention prior to TAVI. After the procedure, invasive systolic peak-to-peak gradient decreased from 70±20 to 5±3 mmHg (p<0.0001). An echocardiogram performed at discharge showed that the aortic valve area (AVA) had increased from 0.67±0.17 to 2.7±0.65 cm2 (p<0.0001), and that the transaortic mean gradient had decreased from 55±21 to 8±3.7 mmHg (p<0.0001).
ADVERSE EVENTS
Two patients experienced cardiac tamponade at the end of the procedure after successful implantation of 31 mm and 29 mm CRS, secondary to perforation of the right ventricle from the temporary pacing wire. Both were treated with efficacious percutaneous subxiphoid drainage and blood transfusion with immediate recovery of haemodynamics. No cases of intraprocedural stroke were observed. Vascular complications occurred in five patients: one patient was treated by transcatheter implantation of a 7/40 mm Fluency® Plus covered stent (Bard Inc., Karlsruhe, Germany), one by ultrasound-guided compression for femoral pseudoaneurysm, and three patients by vascular surgery. In-hospital and 30-day outcomes are displayed in Table 5. Permanent pacemaker (PPM) implantation was performed in 24% (n=19) of the patient population for advanced or complete atrioventricular block (AVB), among whom nine received a 31 mm valve (p=ns). Eleven patients received ≤2 units of packed red blood cells.
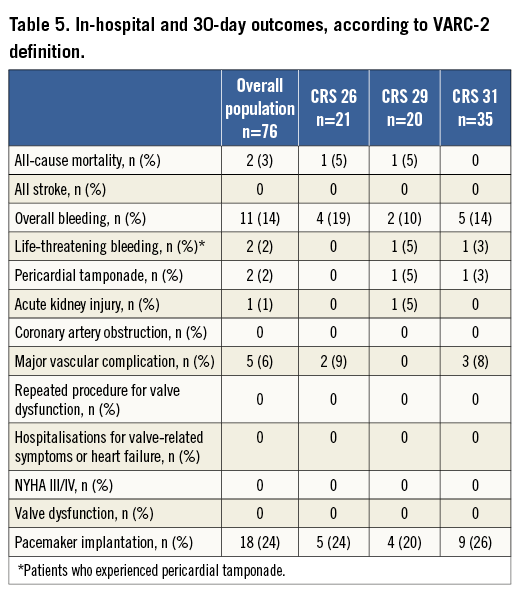
No intra-hospital death occurred, while two deaths (3%) occurred in the first 30 days due to severe pneumonia and sudden cardiac death (Table 5).
CRS 31 MM RESULTS
Patients who received the 31 mm CRS were significantly younger, predominantly male and with a higher risk for cardiac surgery assessed by STS score (Table 2). The mean transvalvular aortic gradient and AVA measured 56 mmHg and 0.66 cm2, respectively, being similar between groups; the mean LVEF was 45%, significantly lower than the 26 and 29 mm groups (Table 3). All patients with LVEF below 35% belonged exclusively to the CRS 31 mm population.
The annulus size measured with TTE/TEE and MSCT was larger in comparison with the 26 and 29 mm groups; the SoV width differed significantly in all three groups, whereas SoV height was significantly different between the 26 and 29 mm, and the 26 and 31 mm cohorts (Table 3). In three patients of the CRS 31 mm group, the transaxillary route was chosen as vascular access on account of severe peripheral vasculopathy. Procedural time, fluoroscopy time and revalving time did not differ (Table 4). At TTE performed at discharge, we observed a similarly significant reduction in transaortic mean gradient and increase in AVA in all three groups; the incidence of paraprosthetic AR was significantly minor in the CRS 31 mm population when compared with the CRS 29 mm population (Table 4). AVB was more frequent, and 26% of patients receiving a 31 mm CRS had PPM implantation (p=ns). In addition, bleeding, vascular and renal complications were similar among groups (Table 5). Duration of hospitalisation did not differ from the other groups (Table 4), and 30-day outcomes were similar (Table 5).
Discussion
We reported on our institutional experience with TAVI after the introduction of the 31 mm CRS device. The overall risk profile assessed by risk scores was substantially similar across the three device sizes in the entire cohort, except for higher STS scores in the CRS 31 mm patients, who were also younger and predominantly male. Forty-six percent of patients included in our analysis received the 31 mm device. This was as a result of the inclusion of patients with larger annuli and more complex aortic root anatomy for TAVI as well as preferential implantation of a larger device when annular measurements were in the borderline zone for 29 or 31 mm devices.
Concerns have been raised about the optimal landing zone and safety of the 31 mm device owing to the increased radial force exerted by a larger inflow portion in the annulus and LVOT9,10. The overall rate of PPM after 31 mm CRS implantation did not differ significantly compared with the other two cohorts, being 26%, 24% and 20% in the CRS 31 mm, 26 mm and 29 mm cohorts, respectively. This is in consonance with published data from other large series where the implantation rate varied from 18 to 30%9-11. For cases of severe AR an oversized device was used preferentially, and yet the PPM implantation did not escalate beyond the mean PPM frequency in the entire population. This might be related to important changes in LVOT geometry that merit consideration in device selection11,12.
The diameters of the constrained parts of both the 29 mm and 31 mm devices are 24 mm, which dispels any incremental risk of coronary ostial compromise with the larger device (Figure 1). Implantation of a 31 mm device can be performed successfully in cases both of aortic stenosis and of regurgitation, with procedural efficacy and safety results that are similar to the 23, 26 or 29 mm valves provided certain pre-procedural and technical expedients are incorporated into the implantation algorithm.
A pre-procedural multimodality imaging evaluation including echocardiography and MSCT is routinely performed in most centres. MSCT has assumed a central role in the pre-procedural evaluation as it allows a better understanding of the aortic annulus dimensions and shape, as well as the LVOT morphology. The manufacturer’s recommendations now include measurement of annulus perimeter to aid in proper device selection. In a recent publication, Buellesfeld et al4 reported on aortic root measurements performed with 3D-MSCT reconstruction in a cohort of aortic stenosis patients before TAVI. They described a perimeter difference of 10%-40% between the LVOT and annulus in 30% of patients included in the cohort. This finding reinforces our strategy of oversizing the CRS device based on dilated left ventricles and when the annular dimensions are in the borderline zone for a 29 mm or 31 mm device.
The technical aspects of the TAVI procedure and the expedients that were incorporated to deploy the 31 mm CRS prosthesis safely in a complex cohort of patients deserve some discussion.
First, we incorporated pre-implantation BAV with an undersized balloon prior to implanting the CRS prosthesis in all cases, except severe AR or a degenerated aortic bioprosthesis. The issue of the necessity of a pre-implantation BAV was raised by Grube et al12. They implanted CRS prostheses in a group of 60 patients. Besides the obvious limitations of sample size, study design and methodology, there was no objective or reproducible assessment of calcification in the aortic valve.
We attest that native aortic valve preparation with BAV performed with an adequately sized balloon is essential to ensure an optimal positioning of the valve in the LVOT and for the stability of the valve during expansion, especially for large devices (31 mm). If the commissures are not fractured/split with BAV, it is likely that the large inflow diameter and high radial force from the prosthesis may cause it to descend deeper into the LVOT in an uncontrolled fashion, leading to implant failure with significant PPR or conduction disturbances. We used a 25 mm BAV balloon for 29 and 31 mm prostheses to limit the number of inflations.
Second, it is critical to ensure optimal positioning of the DCS in relation to the annulus and LVOT before the inflow portion of the valve is released. The landing zone is often difficult to appreciate in cases of large root, hence the DCS should ideally be positioned centrally within the aortic annulus. This may be technically challenging but can often be accomplished by pulling on the stiff wire and the DCS to release the tension in the system. No more than the first horizontal radiopaque marker (4 mm) should be placed below the annular plane prior to initiating the release of the valve frame. The first part of the release (flaring) should be performed under rapid pacing to avoid excessive movement.
The introduction of the 31 mm device has allowed the treatment of patients with severe aortic regurgitation and dilated left ventricles, further expanding the patient population which can receive TAVI.
At present, TAVI for severe AR remains an off-label indication, but several implantations have been performed successfully on a compassionate basis in patients with depressed LVEF and/or at prohibitive risk for cardiac surgery13. Again, meticulous attention to device sizing and implantation is critical to ensure an optimal procedural outcome in these patients. Due to the absence of calcium in the annulus, the device needs to be oversized: thus, for a 21-24 mm annulus a 29 mm CRS, and for an annulus larger than 24 mm a 31 mm CRS are optimal choices. The depth of implantation is 6-8 mm below the annular plane. The sealing is achieved by expansion of the valve frame against the LVOT. The results are often gratifying as valve expansion is optimal providing an excellent sealing against paraprosthetic regurgitation. It is advisable to use rapid pacing at 180-200 bpm during the initial release of the inflow portion (flaring) to arrest the to and fro movement of the DCS in the regurgitant jet.
The device success rate was 92% in our cohort and acute TAV-in-TAV was necessary in five cases, due to low implantation of one CRS 26 mm and four CRS 31 mm prostheses. TAV-in-TAV implantation was accomplished successfully in all cases with immediate and complete resolution of paraprosthetic regurgitation. We submit that TAV-in-TAV is the preferred method for treating implant failure in cases where a 31 mm valve is deployed, as it may not be feasible to pull up and reposition the device with a snare catheter14 due to the high radial force exerted on the LVOT by the inflow portion. TAV-in-TAV is a feasible technique in almost all cases, allowing cases of low implant to be solved effectively15.
Another finding in our experience is that the incidence of paravalvular regurgitation ≥2+ is very low, possibly being the consequence of three factors: better sizing in the diagnostic phase with an MSCT scan, improved implant technique, and the availability of larger valve sizes such as the 31 mm, which allows the reduction of mismatch between the annulus and the device diameter in aortic valves larger than 27 mm.
Our results are different from those published recently by Nijhoff et al16. In this retrospective multicentre registry, 12.3% of all patients treated with TAVI received a CRS 31 mm. In-hospital mortality was 8.5%, and 17% of patients received a second valve for device malposition. These outcomes can be explained by the fact that the study was conducted during the initial phase of CRS 31 mm introduction into clinical use and, despite the very experienced centre involved, there was probably a learning curve effect due to the different characteristics of this size as opposed to the other CRS family devices.
Conclusions
The 31 mm CRS is valuable in that it has provided a truly percutaneous option for the treatment of patients with complex aortic valve disease such as those with large annuli and dilated left ventricles with low ejection fraction. Patients with severe aortic regurgitation can theoretically benefit from this valve size due to its self-expanding properties and due to the large diameter of the inflow part. It can be safely implanted through multiple vascular access sites (transfemoral or transaxillary) with good procedural outcomes and acceptable rates of PPM implantation and vascular complications.
Limitations
The present study is an observational, retrospective, single-centre study. The sample size was relatively small. The reported outcomes may have been influenced by operator experience.
| Impact on daily practice Transcatheter aortic valve implantation is now a consolidated technique to treat inoperable patients with severe aortic stenosis. The CoreValve device is currently available in 23 mm, 26 mm, 29 mm and 31 mm sizes. In daily practice the introduction of the larger size has allowed extending the intervention to patients with uncommon aortic root anatomy, larger annuli with low calcification (as in the case of aortic regurgitation) and dilated left ventricle/LVOT. However, technical tricks are needed in order to optimise the CRS 31 mm implant. |
Conflict of interest statement
G.P. Ussia is a physician proctor for Medtronic Inc. The other authors have no conflicts of interest to declare.
Appendix
Moving image 1. Fluoroscopy during the first steps of implantation of a 31 mm CoreValve Revalving System prosthesis (Medtronic) under rapid right ventricular pacing (150-200 bpm).
Moving image 2. Fluoroscopy during the final phase of deployment of the 31 mm prosthesis.
Moving image 3. Final angiography showing mild aortic regurgitation.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Fluoroscopy during the first steps of implantation of a 31 mm CoreValve Revalving System prosthesis (Medtronic) under rapid right ventricular pacing (150-200 bpm).
Moving image 2. Fluoroscopy during the final phase of deployment of the 31 mm prosthesis.
Moving image 3. Final angiography showing mild aortic regurgitation

