Abstract
The CoreValve Evolut R with EnVeo R delivery catheter is a novel transcatheter heart valve (THV) system with enhanced features that have the potential to improve the safety of transcatheter aortic valve implantation (TAVI). The newly designed delivery catheter is 14 Fr-equivalent and thus expands the option of transfemoral TAVI to a greater proportion of patients. Most importantly, the EnVeo R delivery catheter allows the valve to be recaptured and repositioned during deployment, thus minimising the consequences of THV malposition. Furthermore, the nitinol frame of the CoreValve Evolut R has been redesigned for superior interaction, consistent radial force and optimised cover index across the sizing range, and conformability with the native annulus, thereby hypothetically reducing stress on the left bundle branch. Although large series with long-term follow-up are required to demonstrate the safety and efficacy of this device, we present the first human experience with the Evolut R system.
Introduction
The emergence of second-generation transcatheter aortic valve implantation (TAVI) systems is timely, as this technology is being evaluated in lower-risk patients. Extending the remit of TAVI to lower-risk patients necessitates more flexible, user-friendly and safer devices. Among the TAVI physician’s “wish list” are smaller calibre and more flexible delivery catheters with the ability to recapture, reposition, or remove the transcatheter heart valve (THV) from the body if necessary. The newly designed CoreValve Evolut R™ (Medtronic, Minneapolis, MN, USA) is a low-profile system that encompasses these important requirements. Herein, we describe the technical features of this novel system and describe the first-in-human experience with this device.
CoreValve Evolut R transcatheter heart valve
The CoreValve Evolut R represents a significant step in the evolution of the Medtronic CoreValve family of THVs. The design features of prior iterations have been described elsewhere1, and the Evolut R retains many of the characteristics of its predecessors: a radiopaque self-expanding nitinol support frame, supra-annular trileaflet porcine pericardial leaflets, and porcine pericardium fabric skirt. As before, the Evolut R will be available in 23, 26, 29 and 31 mm sizes.
The cell geometry and frame of the Evolut R have been redesigned to optimise frame interaction with the native anatomy, to improve conformability to the aortic annulus and reduce paravalvular leak (Figure 1). The inflow has more consistent radial force across the sizing spectrum, and the outflow has been shortened and reshaped to provide improved alignment between valve housing and the native sinus, which is expected to reduce stress on the left bundle branch. The EnVeo R catheter valve release mechanism now features a paddle design in place of the previously used tabs, thereby allowing a more consistent release from the delivery catheter. Finally, the valve leaflets are now routinely treated with alpha-amino oleic acid (AOA®) to impede calcium deposition.

Figure 1. The CoreValve Evolut R. The CoreValve Evolut R has been redesigned to provide more consistent radial force across the sizing range with modified cell geometry to improve conformability to the aortic annulus. The new valve release mechanism has a paddle design to prevent hang-ups and the porcine pericardial leaflets are treated with alpha-amino oleic acid (AOA®) to retard mineralisation.
EnVeo R™ delivery catheter with InLine™ sheath
The new EnVeo R delivery catheter (Medtronic, Minneapolis, MN, USA) features a complete redesign of the AccuTrak system (Medtronic) that is currently employed for CoreValve and CoreValve Evolut (Medtronic) implantation (Figure 2). The EnVeo R catheter with InLine sheath (Medtronic) allows the valve to be delivered without the requirement for a separate introducer sheath. As a 14 Fr-equivalent delivery system (true 18 Fr outer diameter) the EnVeo R system represents a 4 Fr reduction in profile compared to the currently used 18 Fr introducer sheaths (approximately 22 Fr outer diameter). Alternatively, the EnVeo R catheter can be introduced through an 18 Fr introducer sheath, if required. The EnVeo R catheter also features a new ergonomic handle that affords more comfortable and stable hand positioning during valve deployment, and an independent mechanism for catheter tip retrieval after implantation. The new system also provides stable and accurate valve deployment by engineering the delivery capsule to be retracted (or advanced) in increments equal to the distance that the deployment wheel is turned (i.e., 1:1 valve deployment). Most importantly, the novel laser-cut nitinol-reinforced capsule provides the ability to resheath or recapture the partially deployed THV (up to 80% of maximal deployment) in order to reposition or retrieve the implant.
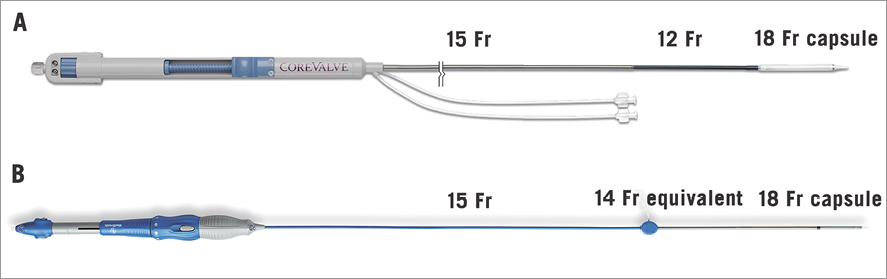
Figure 2. The EnVeo R delivery system. The AccuTrak delivery system (A) has been replaced by the EnVeo R delivery catheter with InLine sheath technology (B). The new system is now a 14 Fr-equivalent system that can deliver the transcatheter heart valve without the requirement for a separate introducer sheath. The modified valve capsule now allows the valve to be fully recaptured and repositioned during deployment.
Clinical applications
In the PARTNER trial (cohorts A and B), implantation of a second THV due to malposition or severe aortic incompetence occurred in 2.47% of cases and is even more common when THVs are used to treat degenerated surgical bioprostheses2,3. Considering the litany of complications associated with THV malposition (mitral valve injury, severe paravalvular leak, conduction abnormalities, THV embolisation) and the independent association with increased one-year cardiovascular mortality3, the capacity to recapture and reposition or remove a malpositioned THV is of considerable importance. The ability to recapture a partially deployed THV also allows the operator to attempt more challenging anatomy, knowing that the system can be retrieved if suboptimal results are encountered. On a cautionary note, attempts to optimise THV implantation by repeatedly recapturing and repositioning the THV could potentially increase the risk of embolic events and could represent a downside to recapturable TAVI systems4. Further study is required to assess this important issue.
Compared to their predecessors, the EnVeo R catheter and InLine sheath represent a significant reduction in the profile of the delivery system. The Evolut R 14 Fr-equivalent system compares favourably to the expanded diameter of the 14-16 Fr eSheath and the 18 Fr sheath used by the Edwards SAPIEN 3 and CENTERA systems (Edwards Lifesciences, Irvine, CA, USA), respectively. Given that major vascular complications are associated with considerable morbidity and mortality4, and that the ratio of the outer diameter of the delivery sheath to the femoral artery (SFAR) is a strong predictor of these complications5, the 4 Fr reduction in sheath size is likely to extend the potential and safety of transfemoral TAVI. Applying the SFAR ratio to the EnVeo R system, transfemoral TAVI can be safely performed in patients with iliofemoral diameters as small as 5.4 mm. Indeed, if the 20% oversizing ratio between the introducer sheath (18 Fr: outer diameter 7.2 mm) and the minimal femoral artery diameter (6 mm) is maintained, then femoral anatomy as small as 5 mm could be navigated with the EnVeo R delivery catheter.
First human experience
The first-in-human experience with the 23 mm CoreValve Evolut R system took place at the McGill University Health Centre in Montreal, Quebec, Canada, in September 2013. The recipient was a 70-year-old female with progressive dyspnoea (NYHA III) due to severely stenotic degenerated aortic bioprosthetic valve (mean gradient 41 mmHg; effective orifice area: 0.61 cm2). The Heart Team considered her too high risk for redo aortic valve surgery as she had previously undergone two coronary artery bypass surgeries and had multiple comorbid medical conditions (Society of Thoracic Surgeons mortality risk score: 7.9%). The degenerated aortic bioprosthesis (19 mm Carpentier Edwards Perimount Magna Ease) had an internal stent diameter of 16.5 mm on multislice computed tomography (Figure 3A and Figure 3B). The 23 mm Evolut R is suitable for native annuli between 18 and 20 mm in diameter; however, smaller diameter degenerated surgical bioprostheses have been successfully treated6. The CoreValve Evolut R was successfully implanted via the right femoral artery using the EnVeo R delivery catheter and InLine sheath (Figure 3C - Figure 3E) (Moving image 1). In this case, it was not necessary to recapture the Evolut R during deployment. Post implantation, the peak-to-peak and mean transaortic valve gradients were gratifyingly low: 6 mmHg by invasive pressure recording and 8 mmHg by echocardiography, respectively (Figure 3F). It is likely that the supra-annular position of the CoreValve Evolut R leaflets accounted for this low post-procedural gradient. There was no central or paravalvular aortic regurgitation.
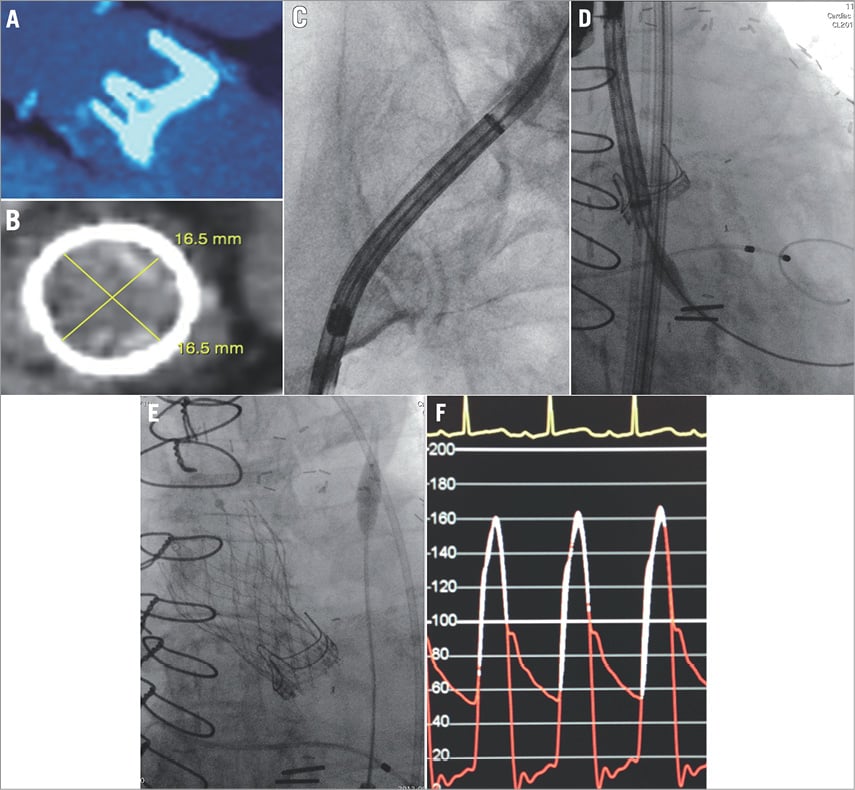
Figure 3. CoreValve Evolut R: first-in-human experience. The first-in-human CoreValve Evolut R was implanted inside a 19 mm Carpentier Edwards Perimount Magna Ease (manufacturer labelled internal stent diameter 17.5 mm) (A). The internal stent diameter of the degenerated aortic bioprosthesis was 16.5 mm on multislice computed tomography (B). The EnVeo R delivery system was advanced through the tortuous calcified iliofemoral anatomy (C), and the CoreValve Evolut R was positioned appropriately. The final position of the Evolut R was acceptable (E), and the post-implantation transvalvular gradient decreased from 41 mmHg to 6 mmHg (F).
Limitations
Herein, we describe the first human experience with the CoreValve Evolut R. Although this device has been specifically designed to improve procedural outcomes, demonstration of device safety and clinical efficacy requires verification. For example, the potential to reduce the repeated attempts to position the THV optimally could potentially increase the risk of embolic events. To this end, the results of the ongoing CoreValve Evolut R Clinical Study (ClinicalTrials.gov Identifier: NCT01876420) are eagerly awaited.
Conclusions
The CoreValve Evolut R is a low-profile THV system that will extend the possibility of transfemoral TAVI to more patients. Coupled with the ability to recapture and reposition the THV, the CoreValve Evulot R has the potential to improve the accuracy and safety of TAVI procedures.
Impact on daily practice
The redesigned CoreValve Evolut R system should impact daily clinical practice in several ways. First, the 4 Fr reduction in the diameter of the EnVeo R delivery catheter should extend the possibility of transfemoral TAVI to a greater proportion of patients. It is possible that patients with minimal femoral artery diameters as small as 5 mm could become transfemoral TAVI candidates. Second, the new delivery system allows one to one release or recapture of the THV, thereby providing for more stable valve implantation. Most importantly, the ability to recapture and reposition the CoreValve Evolut R during deployment is expected to improve the safety of TAVI. This important feature will potentially reduce THV malposition, embolisation, and the requirement for implantation of multiple THVs.
Conflict of interest statement
N. Piazza is a consultant for Medtronic. G. Martucci is a proctor for Medtronic. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. First-in-human CoreValve Evolut R implantation.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1.

