Abstract
Transcatheter aortic valve implantation (TAVI) has evolved from an exclusive, highly complex and hazardous procedure into a mature, safe and streamlined therapy for patients with severe aortic stenosis (AS). Various successive device iterations and product refinements have created a dynamic and competitive field with a spectrum of different CE-marked transcatheter heart valve (THV) designs. This review provides a practical overview of current CE-marked THVs with a focus on respective sizing algorithms and delivery strategies.
Introduction
Transcatheter aortic valve implantation has evolved from an exclusive, highly complex and hazardous procedure into a mature, safe and streamlined therapy for patients with severe aortic stenosis. TAVI uptake and adoption have increased exponentially and the annual TAVI rate has already surpassed SAVR in selected nations1. More liberal patient selection and the exploration of new indications, such as asymptomatic severe AS, moderate AS with heart failure and aortic regurgitation, may further broaden the TAVI landscape. This remarkable clinical success has stimulated successive device iterations and product refinement, resulting in a dynamic and competitive field with a spectrum of different CE-marked THV designs2. This review provides a practical overview of current CE-marked THVs with a focus on respective sizing algorithms and delivery strategies.
Overview of CE-marked transcatheter heart valves
MEDTRONIC EVOLUT R
The CoreValve® Evolut™ R device (Medtronic, Minneapolis, MN, USA) consists of a trileaflet porcine pericardial valve housed in a nitinol self-expanding frame and builds on the properties of its CoreValve predecessor3. A fluoroscopic image of the Evolut R is shown in Figure 1A. The valve leaflets are in a supra-annular position to maximise the effective orifice area and the redesigned nitinol frame has a larger cell size with a smaller frame height of 45 mm. Its inflow has a more consistent radial force to achieve optimal conformation to the aortic annulus. The mid segment is narrower and the outflow segment abuts the aortic wall above the sinotubular junction for improved alignment between valve housing and the native sinus. A 12 mm porcine pericardium fabric skirt surrounds the inflow segment and is continuous with the valve leaflets to protect against paravalvular leakage. The valve is fully repositionable and retrievable up to approximately 80-90% of total deployment. Three valve sizes are currently available covering a range of aortic annular diameters from 18 to 26 mm (Figure 2). The novel EnVeo™ delivery system and integrated 14 Fr InLine™ sheath (Medtronic) have significantly reduced the overall profile and are compatible with vessel sizes 5 mm and above. This smaller profile makes a transfemoral approach possible for a wider spectrum of patients, including those with more challenging iliofemoral anatomy including small, tortuous or atherosclerotic vessels.
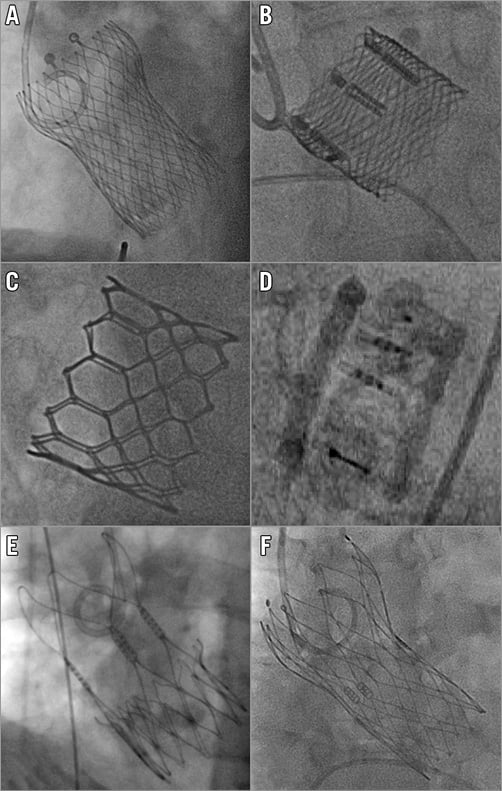
Figure 1. Fluoroscopic views of new-generation transcatheter heart valves after implantation. A) Medtronic Evolut R. B) Boston Lotus. C) Edwards SAPIEN 3. D) Direct Flow Medical. E) Symetis ACURATE. F) St. Jude Portico.
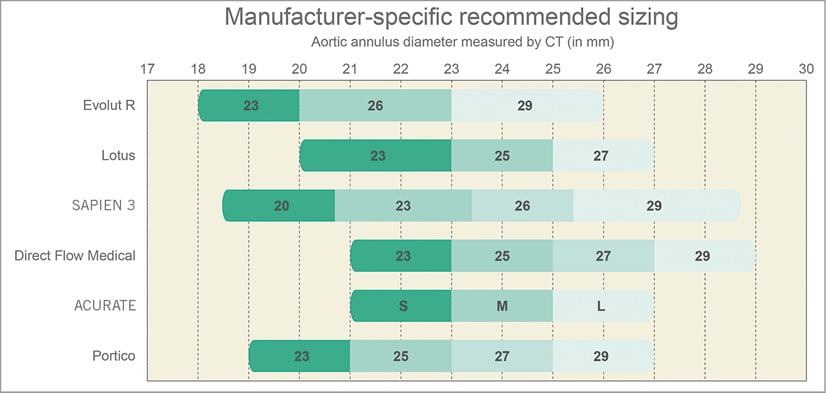
Figure 2. Combined sizing chart of new CE-marked transcatheter heart valves.
BOSTON LOTUS
The Lotus™ valve system (Boston Scientific, Marlborough, MA, USA) is a trileaflet bovine pericardial valve supported on a braided nitinol frame which is deployed through active mechanical expansion, gradually decreasing in size from 70 mm (fully sheathed) to 35 mm upon unsheathing and 19 mm at release (Figure 1B). The nitinol frame expands upon unsheathing and valve function is almost instant. A central radiopaque marker facilitates positioning of the prosthesis within the aortic root. The inflow segment is covered with an adaptive seal to conform to aortic root irregularities and reduce paravalvular leakage. The delivery catheter is attached to the bioprosthesis with three coupling fingers. The valve has a unique locking mechanism that connects the posts to the corresponding buckles and is completely deployed at this point. Valve function and position can be assessed in terms of implantation depth, paravalvular leakage, and location relative to the coronary ostia, and can still be fully repositioned or retrieved when deemed necessary. The Lotus valve comes in three sizes, covering a range of annular diameters from 19 to 27 mm (Figure 2). The delivery system has an 18-20 Fr profile and requires a minimum vessel size of 6 mm.
EDWARDS SAPIEN 3 VALVE
The SAPIEN 3 represents the fourth iteration in the balloon-expandable SAPIEN series (Edwards Lifesciences, Irvine, CA, USA)4. The cobalt-chromium frame houses three bovine pericardial leaflets and has a polyethylene terephthalate (PET) skirt at its inflow portion and an outer PET sealing cuff to reduce paravalvular leakage (Figure 1C). The novel Commander delivery system consists of an outer deflectable flex catheter and an inner balloon catheter with radiopaque alignment markers. A central radiopaque balloon marker and an additional small wheel for fine alignment of the transcatheter heart valve increase accuracy in positioning. The valve is loaded on the balloon in the abdominal aorta, which helps downsize the overall introduction profile. Furthermore, a dedicated 14 or 16 Fr expandable eSheath temporarily expands as the device passes through the iliofemoral vessels (minimum diameter 5.5 mm) and then recoils to its smaller calibre. There are four available valve sizes to accommodate aortic annular diameters ranging from 18.5 to 29.5 mm (Figure 2).
DIRECT FLOW MEDICAL
The Direct Flow Medical® device (Direct Flow Medical, Santa Rosa, CA, USA) is an 18 Fr compatible trileaflet bovine pericardial valve attached to a non-metallic frame covered by a polyester fabric (Figure 1D)5. Flexible pillars connect the aortic and ventricular rings which are inflated with a radiopaque solution. The system is unsheathed in the left ventricle followed by inflation of the ventricular ring and retraction of the device into the aortic root. Three positioning wires allow final manipulation before inflation of the aortic ring. The device can be repositioned or retrieved by deflating both rings if device depth, proximity to the coronaries or residual paravalvular leakage are unsatisfactory. Once the correct device position is confirmed, a quick curing polymer is instilled instead of the radiopaque solution to secure permanent implantation and the valve is released by detaching the positioning wires. The Direct Flow Medical valve comes in four sizes, covering annular diameters from 21 to 29 mm (Figure 2).
SYMETIS ACURATE
The ACURATE TA™ (for transapical) and neo™ (for transfemoral) valves (Symetis SA, Ecublens, Switzerland) consist of a nitinol self-expanding frame with three stabilisation arches at the distal/aortic edge, an upper and a lower crown (Figure 1E)6. The lower inflow crown is covered by a polyethylene terephthalate sealing skirt while the upper crown segment provides supra-annular anchoring and houses three pericardial leaflets (ACURATE neo supra-annular; ACURATE TA intra-annular). Transfemoral deployment follows a top-down approach. The upper crown is released first to capture the native leaflets followed by release of the stabilisation arches and unsheathing of the lower crown. There is no need for rapid right ventricular pacing during deployment. During transapical deployment, the stabilisation arches and upper crown are released first before pulling the system down to embrace and compress the native leaflets. The lower crown is then unsheathed and self-detaches from the delivery system. There are three available valve sizes covering annular diameters from 21 to 27 mm (Figure 2), and the delivery system fits within an 18 Fr transfemoral sheath.
ST. JUDE PORTICO
The Portico™ device (St. Jude Medical, St. Paul, MN, USA) is an intra-annular trileaflet bovine pericardial valve housed in a nitinol self-expanding frame with a height of 47 mm7. The tubular inflow portion (9 mm height) has a porcine pericardial sealing cuff and the outflow segment (38 mm height) consists of large cells extending the frame towards the ascending aorta to provide stability (Figure 1F). The Portico is fully repositionable and resheathable until approximately 85% of deployment. Implantation starts with expansion and sealing of the inflow segment, with the valve functioning early during deployment. There are four available sizes for annular diameters ranging from 19 to 27 mm (Figure 2). The transfemoral delivery system is a flexible 18 Fr (for smaller valve sizes) or 19 Fr catheter (for larger sizes). The valve can be implanted using dedicated sheaths or via a 19 Fr SoloPath™ sheath (Terumo Europe NV, Leuven, Belgium).
Delivery strategies
The transfemoral route is the access site of first choice for transcatheter aortic valve delivery and has consistently shown the best outcomes, especially compared to the transapical approach8. The smaller profile of latest-generation devices has increased the proportion of patients who are eligible for femoral access with a minimum required vessel size currently down to 5 mm. Nevertheless, selected patients may fare better with an alternative access route due to excessive calcification, atherosclerotic disease, tortuosity or insufficient vessel calibre. Various options exist, including transapical, trans-subclavian/axillary, transcarotid, direct aortic and, more recently, transcaval.
Transapical delivery is possible for SAPIEN 3 and ACURATE TA and the self-expanding systems are compatible with trans-subclavian/axillary access. Direct aortic TAVI can be performed with virtually any device platform and the transcarotid route has been successfully applied in selected centres. The transcaval technique is the newest way of bypassing heavily diseased iliac arteries but requires a non-calcified segment of the abdominal aorta9. Briefly, the inferior vena cava is connected with the abdominal aorta by puncturing through the venous and arterial walls with a 0.014” “chronic total occlusion” stiff coronary guidewire. Upon entering the aorta, this guidewire is snared and eventually replaced by a stiff 0.035” guidewire. The TAVI procedure is then completed as a typical retrograde transfemoral procedure. After valve delivery, the aorto-caval communication is typically closed with St. Jude AMPLATZER™ nitinol closure devices (VSD or PDA occluders). The fate and adoption of alternative access strategies remains unclear. Indeed, relatively straightforward execution without the need for general anaesthesia is arguably the most attractive feature of the standard retrograde transfemoral approach. Multislice computed tomography (MSCT) angiography is currently the preferred imaging tool for comprehensive assessment of the entire arterial tree relevant to TAVI. Conventional angiography may provide additional information given its superior spatial resolution or when CT imaging is suboptimal. A major limitation of ultrasound examination is its inability to visualise the retroperitoneal space.
Valve size selection
Pre-procedural imaging planning is key for any successful TAVI programme. Access site, transcatheter heart valve and consequent device sizing typically rely on MSCT (Figure 3). Inaccurate sizing may have important clinical consequences. Undersizing can result in prosthesis-patient mismatch, paravalvular leakage, device migration and embolisation, all of which can adversely influence prognosis after TAVI. Conversely, oversizing may lead to annular rupture, prosthesis underexpansion with subsequent risk of central transvalvular regurgitation, and conduction abnormalities due to excessive compression of the conduction system in the LVOT. An integrated sizing chart for current CE-marked THVs is displayed in Figure 2. Of note, self-expanding devices typically require more oversizing relative to the native anatomy, as opposed to closer matching with the SAPIEN 3, Direct Flow and Lotus devices. Several imaging modalities are available to measure native annular dimensions and help guide THV size selection.
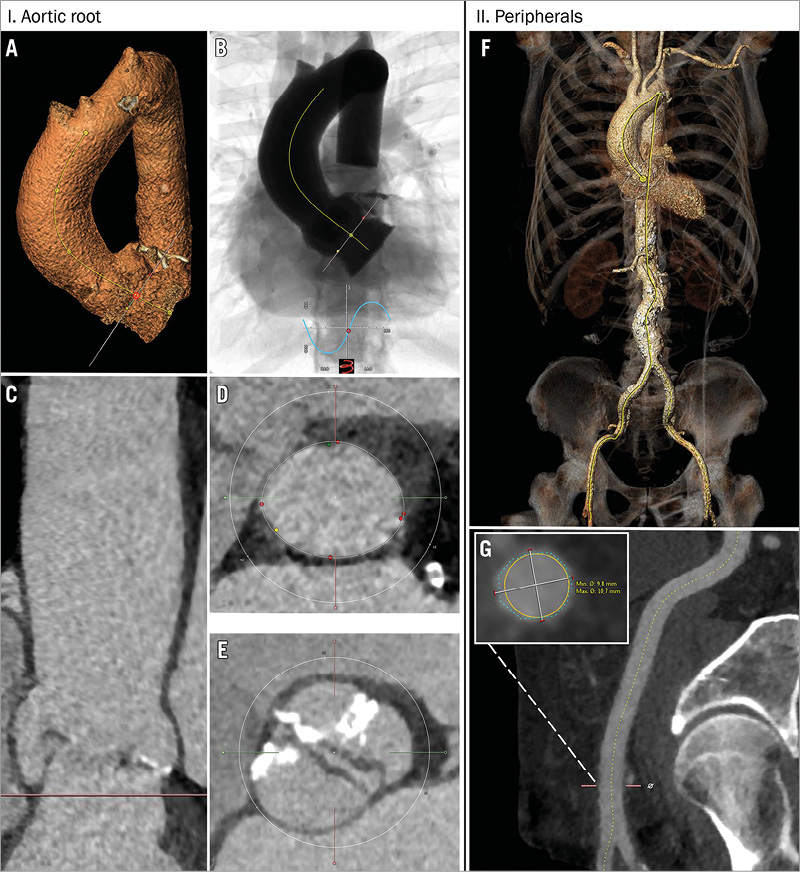
Figure 3. Stepwise analysis of multislice CT with automatic 3mensio software. A) Segmentation and centrelining of aortic root. B) Prediction of optimal C-arm projection. C) Aortic root in a longitudinal view which can be rotated 360 degrees for appreciation of root dimensions and distance to coronaries. D) Smooth tracing of the annular border for calculation of perimeter, area and mean diameters. E) Appreciation of the aortic valve anatomy (in this case a bicuspid aortic valve). F) Three-dimensional reconstruction of peripheral arteries and aorta. G) Estimation of tortuosity and calcium load in the common femoral artery with cross-sectional measurements of vessel diameters at any level.
MULTISLICE COMPUTED TOMOGRAPHY (MSCT)
A single MSCT study can offer vital information concerning the aortic root and entire relevant arterial trajectory, including the aorta, subclavian and iliofemoral system. MSCT has become the standard for 3D assessment of the aortic root in terms of dimensions, calcium distribution and height of the coronary ostia relative to the virtual annulus10. Various dedicated software packages (3mensio [Pie Medical Imaging, Maastricht, The Netherlands], Philips Heart Navigator [Philips Medical Systems, Eindhoven, The Netherlands], Siemens syngo Aortic Valve Guide [Siemens AG, Munich, Germany] and GE Innova [GE Healthcare Ltd, Little Chalfont, Buckinghamshire, UK]) simplify the process of multiplanar reconstruction and make these advanced analyses accessible to interventionalists without extensive radiological training. Interestingly, sizing charts for self-expanding systems rely on aortic annular perimeter (and derived diameters) whereas area is the preferred parameter for balloon and mechanically expandable THVs. Derived diameters will not differ much in principle although the perimeter is less influenced by anatomic variation during the cardiac cycle and may result in larger derived diameters.
ECHOCARDIOGRAPHY
Transthoracic echocardiography is not as accurate as transoesophageal echocardiography and 2D echocardiography requires expert interpretation to ensure that the aortic annulus is viewed in the correct plane to avoid underestimation of minimum and maximum diameters. The advent of 3D echocardiography may fix this important limitation, although the spatial resolution of 3D TOE remains inferior to MSCT and aortic calcification may negatively affect imaging quality. Controversy surrounds the correlation between 3D TOE and MSCT sizing. Importantly, TOE quality depends on the experience of the echocardiographer and is characterised by wider inter-observer variability. In a recent study, aortic annulus measurements were significantly smaller with TOE versus MSCT, particularly in more oval annular anatomy11. Three-dimensional TOE is a valid alternative to MSCT in patients with severe renal insufficiency (to avoid contrast exposure) or a suboptimal MSCT study (e.g., motion artefacts, out-of-phase contrast injection).
MAGNETIC RESONANCE IMAGING
Cardiac magnetic resonance imaging (CMR) is a valid alternative to MSCT for device sizing. CMR is effective to determine aortic root dimensions and evaluate thoracic arterial structures yet struggles with calcium and cannot be used in patients who have an MRI non-compatible pacing device or are claustrophobic.
OTHER MODALITIES
Rotational angiography (R-angio) is a novel technique performed in the catheterisation laboratory using simultaneous C-arm rotation and diluted contrast injection. R-angio provides CT-like reconstruction of the aortic root with accurate sizing; however, patient characteristics (e.g., BMI >29 kg/m2) and a significant learning curve hamper its adoption in clinical practice12.
Simultaneous balloon aortic valvuloplasty and supra-aortic contrast angiography may help in root sizing and demonstrate the interaction of native aortic valve leaflets with the coronary ostia. This ancillary technique may be particularly helpful when MSCT sizing is ambiguous between two consecutive device sizes13,14.
Conclusion
Multiple CE-marked options for TAVI exist and MSCT is now the cornerstone to determine the optimal selection of the appropriate access site, transcatheter valve design and size.
Funding
The Erasmus Medical Center has received research grants from Claret Medical, Boston Scientific, Medtronic and Edwards Lifesciences.
Conflict of interest statement
H. Möllmann is a speaker and has received proctor honoraria from Abbott, Edwards Lifesciences, St. Jude Medical and Symetis. A. Latib is a consultant for Medtronic and Direct Flow Medical. D. Tchetche is a proctor for Edwards Lifesciences, Boston Scientific and Medtronic. G. Manoharan is a proctor for St. Jude Medical. The other authors have no conflicts of interest to declare.

