Abstract
The Ross operation is the procedure of choice for aortic valve disease in paediatric patients, because of the potential for growth of the pulmonary autograft and because anticoagulation is not required. However, early and late autograft dilatation and severe aortic regurgitation may occur. Transcatheter aortic valve implantation (TAVI) is an effective procedure for treatment of severe degenerative aortic stenosis in patients deemed inoperable or high surgical risk. Off-label treatment of severe non-calcified aortic regurgitation with transcatheter heart valves has occasionally been reported.
We describe the first case of TAVI for severe aortic regurgitation in a young woman 10 years after a Ross operation. The procedure was performed on a compassionate basis after the patient was deemed inoperable because of severe reactive pulmonary hypertension (95/55/68 mmHg; pulmonary resistance 18.3 UR) and haemodynamic compromise. A 29 mm CoreValve™ (Medtronic, Minneapolis, MN, USA) was implanted. A second prosthesis was deployed “valve-in-valve” for residual severe paravalvular leak, caused by the peculiar post-surgical anatomy of the left ventricular outflow tract. The procedure was successful and the in-hospital course was uncomplicated. During follow-up, pulmonary pressure and resistances were significantly lowered and at four years the patient showed markedly improved exercise tolerance.
Introduction
Aortic valve replacement with pulmonary autograft was first described by Ross in 1967 as an alternative to mechanical aortic valve replacement in paediatric patients1. Indeed, the ability of the autograft in the aortic position to accommodate growth and freedom from chronic anticoagulation make the Ross operation the preferred therapeutic option for the infant and child with aortic valve disease or complex left heart obstruction. Late outcome for the Ross procedure is excellent in terms both of survival and of quality of life2. However, late root dilatation and autograft regurgitation necessitating reoperation may occur and portend a worse prognosis3.
Transcatheter aortic valve implantation (TAVI) is a safe and effective procedure for the treatment of severe degenerative aortic stenosis (AS) in patients deemed inoperable or high surgical risk4.Non-calcified aortic regurgitation has typically been considered a contraindication for TAVI. However, a few cases of compassionate off-label treatment of severe non-calcified aortic regurgitation with transcatheter heart valves have been reported5,6.
We describe the first successful TAVI procedure for severe autograft regurgitation and reactive pulmonary hypertension 10 years after a Ross procedure.
Case description
A 23-year-old woman was admitted to our hospital with symptoms and signs of biventricular heart failure. Her clinical history included a neonatal diagnosis of severe AS treated with surgical transventricular valvulotomy. When she was four years old, recurrent AS was treated with percutaneous balloon aortic valvuloplasty. Nine years later she underwent a Ross operation, with insertion of a pulmonary autograft into the aortic root, re-implantation of the coronary arteries and restoration of the right ventricle outflow tract with a pulmonary homograft to replace the pulmonic valve and the main pulmonary artery. Five years later she presented effort dyspnoea New York Heart Association (NYHA) functional Class II. A diagnosis of post-capillary pulmonary hypertension associated with moderate aortic regurgitation (AR) was made. Pulmonary artery pressure was 97/38/63 and pulmonary capillary wedge (PCW) pressure 42 mmHg. Medical treatment was initially effective to control symptoms and to decrease pulmonary pressure. A few years later, however, her clinical status rapidly deteriorated and she was referred to our pulmonary hypertension centre. Echocardiography showed severe AR, severe left atrial enlargement, pulmonary hypertension, moderate right ventricle (RV) enlargement, and reduction of RV function (Figure 1). The homograft in the pulmonary position was functioning well without significant gradient and/or regurgitation. Right heart catheterisation showed moderate reactive post-capillary pulmonary hypertension (63/32/41 mmHg; PCW 19 mmHg; pulmonary resistance 7.6 UR), reduced cardiac output (2.9 L/min) and reduced cardiac index (1.6 L/min/m2). Aortic angiography confirmed dilatation of the autograft and severe aortic regurgitation (Figure 2 and Moving image 1). The patient was referred for surgical aortic valve replacement but she decided to refuse the procedure and was discharged home with full medical treatment.
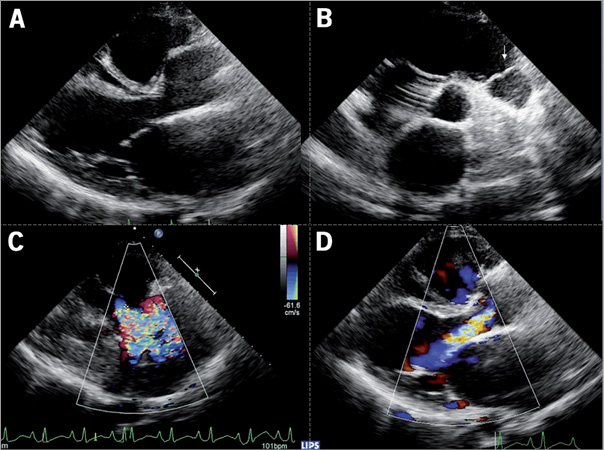
Figure 1. Echocardiography before the procedure. A) Morphology of the left ventricle outflow tract and aortic root in the parasternal longitudinal view. B) Short-axis view showing the pulmonary homograft (arrow). C) and D) Echo colour Doppler imaging showing severe autograft regurgitation.
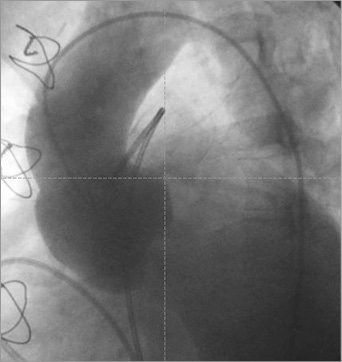
Figure 2. Baseline aortography showing the morphology of the aortic root and severe autograft regurgitation.
In June 2009 she was readmitted urgently to our hospital for congestive heart failure. Even after maximised medical treatment, pulmonary artery pressure was 95/55/68 mmHg, PCW 24 mmHg, pulmonary resistance 18.3 UR, right atrium mean 12 mmHg, cardiac output 2.4 L/min and cardiac index 1.4 L/min/m2. The cardiac surgeon deemed the patient inoperable on the basis of the important haemodynamic compromise, severe pulmonary hypertension and the difficult prediction of functional recovery because of the very high reactive pulmonary resistance. The option of combined heart and lung transplantation was considered but finally rejected because of the unstable clinical status. After a multidisciplinary meeting, we decided to perform a compassionate TAVI. The procedure was approved by an institutional review committee and the subject gave informed consent.
Differently from the current standards, the procedure was undertaken in a hybrid room with on-site surgical back-up, under general anaesthesia. The annulus size measured 25 mm, hence a 29 mm Medtronic CoreValve™ (Medtronic, Minneapolis, MN, USA) was implanted with a transfemoral approach (Figure 3, Moving image 2-Moving image 4). After bioprosthesis release, angiography, transoesophageal echocardiography (TEE) and pressure tracings showed severe aortic regurgitation. The TEE showed the regurgitation to be entirely paravalvular and suggested that the prosthesis could be too high with respect to the “true” left ventricle outflow tract. Therefore, in an attempt to seal the predominantly paravalvular leak we decided to implant a second bioprosthesis, “valve-in-valve”, deeper into the left ventricle (Figure 4, Moving image 5, Moving image 6). After the release of the second valve, diastolic pressure rose to 70 mmHg, the dicrotic notch reappeared on the haemodynamic tracing, and both aortography and TEE showed trivial valve regurgitation and patency of both coronary ostia.
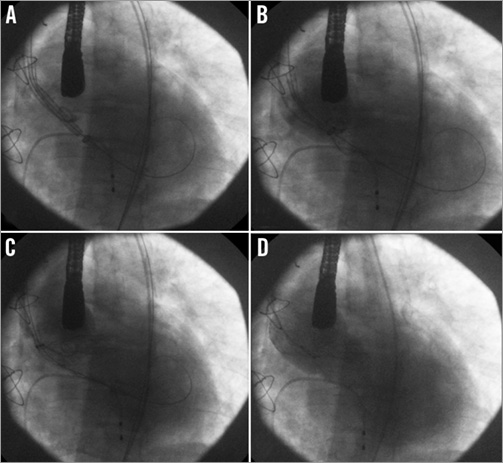
Figure 3. Implantation of the first CoreValve™ bioprosthesis. A) Positioning of the device. The pigtail is positioned on the right coronary cusp and its lower part corresponds approximately to the aortic annulus. The prosthesis was advanced 6 to 8 mm below the annulus. B) Release of the first third of the device. C) Release of the first two thirds of the prosthesis. D) Angiographic control after the complete release of the bioprosthesis showing persistent severe regurgitation.
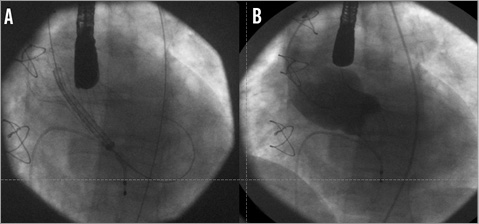
Figure 4. Angiographic assessment of the second bioprosthesis. A) Positioning of the second device 4 to 6 mm lower than the first one. B) Final aortography showing the virtual absence of residual regurgitation and the particular appearance of the “new” aortic root.
The hospital course was uneventful, with immediate symptomatic relief and functional improvement. Transthoracic echocardiography confirmed the good function of the prosthesis in the absence of significant residual regurgitation, the dramatic reduction of tricuspid regurgitation and the decrease to 50 mmHg of estimated systolic pulmonary artery pressure. The ECG revealed mild prolongation of the QRS complex with pre-existing left bundle branch block morphology. No episodes of atrioventricular block or bradycardia were detected during 48 hours continuous ECG monitoring. The patient was discharged home two weeks after the procedure.
Three years after TAVI, cardiac catheterisation confirmed the persistent reduction of pulmonary artery pressure (48/19/31 mmHg) and pulmonary vascular resistance (3.8 UR), with normalised cardiac index at rest (2.6 L/min/m2). Echocardiography demonstrated good functioning of the valve (trivial AR, AVA 1.6 cm2, max gradient 13 mmHg, mean 7 mmHg), reduced volumes and normalisation of right ventricular function (Moving image 7-Moving image 9). At four-year clinical follow-up, the patient showed improved exercise tolerance at the six-minute walk test and a NYHA functional Class I.
Discussion
This case demonstrates a possible novel frontier of transcatheter heart valve techniques. TAVI has been developed to treat inoperable patients and patients at high surgical risk with severe degenerative aortic stenosis. Nevertheless, other possible applications have progressively emerged, including treatment of degenerated surgical bioprosthesis7, stenotic bicuspid aortic valve8, and non-calcified aortic regurgitation5,6. Isolated aortic regurgitation is a technical challenge. The absence of calcification and the frequent coexisting aortic root dilatation make the anchorage of the prosthesis more difficult, with a consequent increased risk of device migration. The CoreValve™ prosthesis is generally preferred for this indication, because of the self-expanding metallic frame which may progressively adapt to the aortic root and the supposed additional stabilisation provided by the aortic portion of the stent. In these cases, a 10% to 20% oversizing of the self-expanding valve with respect to the annulus perimeter is generally recommended when choosing the prosthesis size5. New valve designs may prove to be more safe and effective to treat pure aortic regurgitation. Recently, the JenaValve (JenaValve Technology GmbH, Munich, Germany) has been proposed as a better option in these specific patients due to its stent design which provides clipping of the native aortic valve leaflets6. In addition, a few valve prototypes dedicated to treatment of aortic regurgitation are under development and will probably be available shortly.
Transcatheter heart valve replacement is a growing field for congenital heart diseases. Percutaneous pulmonary valve implantation has been shown to be feasible, safe and effective9. With the present case, we showed the feasibility and efficacy of TAVI to treat autograft dysfunction after a Ross operation, with good procedural success and long-term clinical benefit. However, several technical issues still need to be addressed. From the careful review of this case it is not clear whether or not the implant of the first prosthesis directly at a lower level, i.e., much lower than the autograft valve, could have worked out in the first instance. If so, the implantation technique would differ significantly from the technique commonly used for native valve stenosis in which the new valve is situated at a supra-annular level. In our patient, the valve within the second prosthesis was clearly in a subannular position. Multimodality imaging should be mandatory for the careful analysis of the modified post-surgical anatomy and should be used for better procedural planning.
Transcatheter valve implantation for pulmonary homograft failure after a Ross operation has also been described. This procedure has been shown to be feasible and effective up to three years of follow-up in a small series of patients10, providing a further demonstration of the large horizons of transcatheter procedures for both acquired and congenital heart diseases.
Conclusion
TAVI for severe autograft regurgitation after a Ross operation is feasible, and in our patient was able to achieve excellent haemodynamic and clinical results. Thus, it might help to reduce the need for reoperation in these patients. However, several technical limitations must first be resolved. Until then, the procedure must be considered only as a last-option therapeutic strategy for really inoperable patients.
Conflict of interest statement
JC. Laborde is a physician working as proctor for Medtronic. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. The dilated autograft and severe aortic regurgitation.
Moving image 2. The TAVI procedure: starting position.
Moving image 3. The TAVI procedure: partial release.
Moving image 4. The TAVI procedure: final result, with severe aortic regurgitation.
Moving image 5. The second valve-in-valve implantation: starting position.
Moving image 6. The second valve-in-valve implantation: final result showing trivial residual aortic regurgitation.
Moving image 7. Three-year echocardiography: parasternal longitudinal view, without evidence of significant aortic regurgitation.
Moving image 8. Three-year echocardiography: short-axis view, without evidence of paravalvular aortic regurgitation.
Moving image 9. Three-year echocardiography: four-chamber view showing normalised right ventricular volume and systolic function.

